
Iodination reactions with aluminum iodide: Discussion series on bromination/iodination reactions 27
Iodides are essential as intermediates, with applications ranging from active pharmaceutical ingredients (APIs) to antibacterial agents and electronic materials. Various iodinating agents, such as elemental iodine and alkali iodides, are used according to the target product when synthesizing iodides.
In this article, we give an overview of one such iodinating agent: aluminum iodide.
Aluminum iodide excels as an iodinating agent that solves a particular set of iodination reaction issues. In turn, basic knowledge of aluminum iodide is a valuable part of learning about iodination reactions.
contents
Aluminum iodide
Aluminum iodide (Al2I6) is a slightly yellow powder that is sensitive to moisture. It has a melting point of 191°C and a density of 3.98 g/cm3. While commercially available, it is relatively expensive, unlike Al2Cl6. For this reason, aluminum iodide is often used via generation in situ by reacting aluminum powder with iodine.
Iodination reactions with aluminum iodide
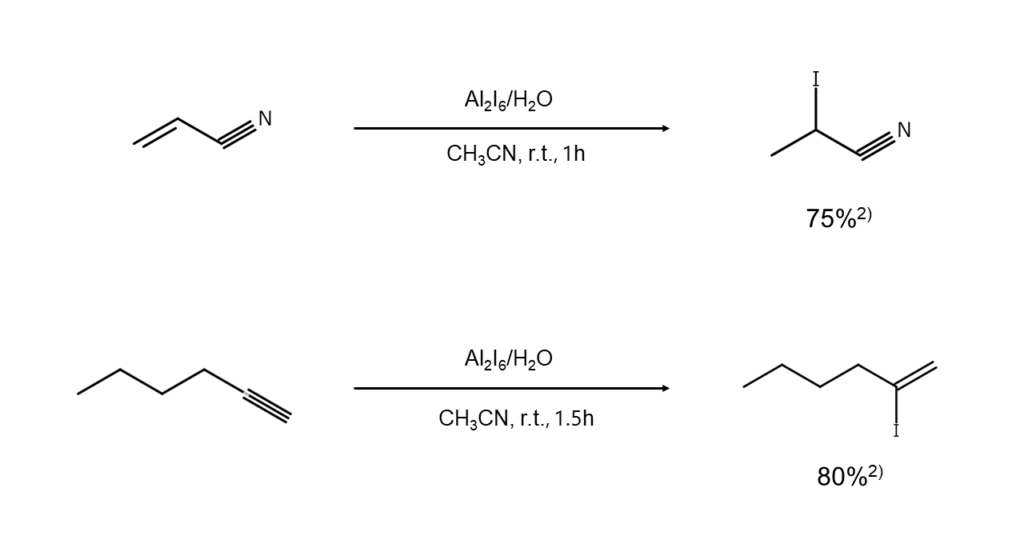
① Alkene and alkyne hydroiodation
Aluminum iodide is a particularly useful reagent in the hydroiodation of alkenes and alkynes. The following illustrates reaction examples.
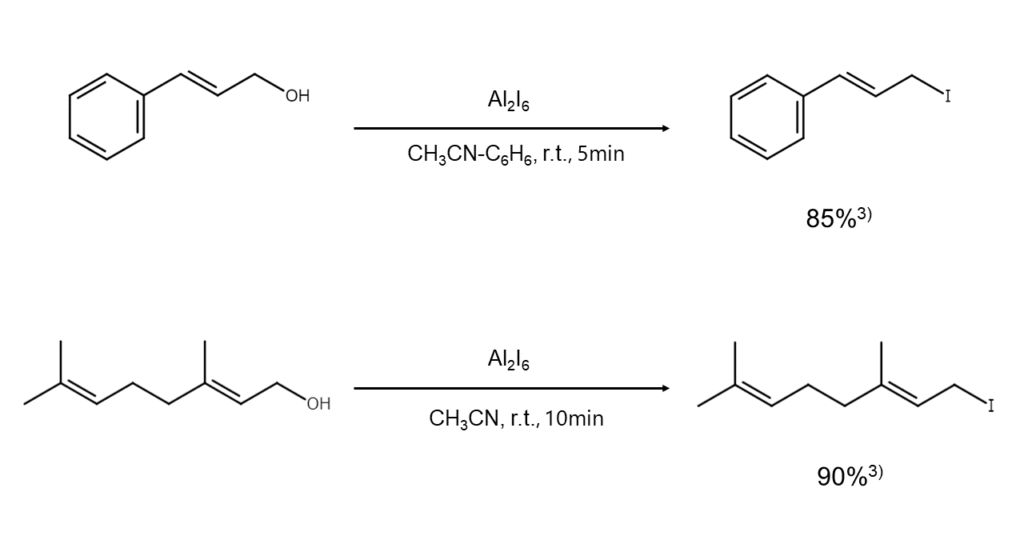
② Conversion of alcohols into iodoalkanes
The conversion of an alcohol into an iodoalkane generally involves an alkali iodide and inorganic acid combination or the use of HI. However, this approach presents a problem. Excessive amounts of reagents are required to achieve good results, which translates into significant material wastage. There are further issues found with this approach under acidic conditions, including the isomerization of carbon skeletons and side reactions induced by small amounts of iodine generated during the reaction process.
The solution comes in the form of aluminum iodide, an exceptionally excellent reagent that offers a way around these challenges.
Tertiary, allyl, and benzyl alcohols react rapidly when using aluminum iodide. Caution is required, however, as reactions progress slowly for primary and secondary alcohols, and vic-diols easily undergo deoxygenation and conversion into an alkene.
Examples of reactions that use aluminum iodide are shown below.
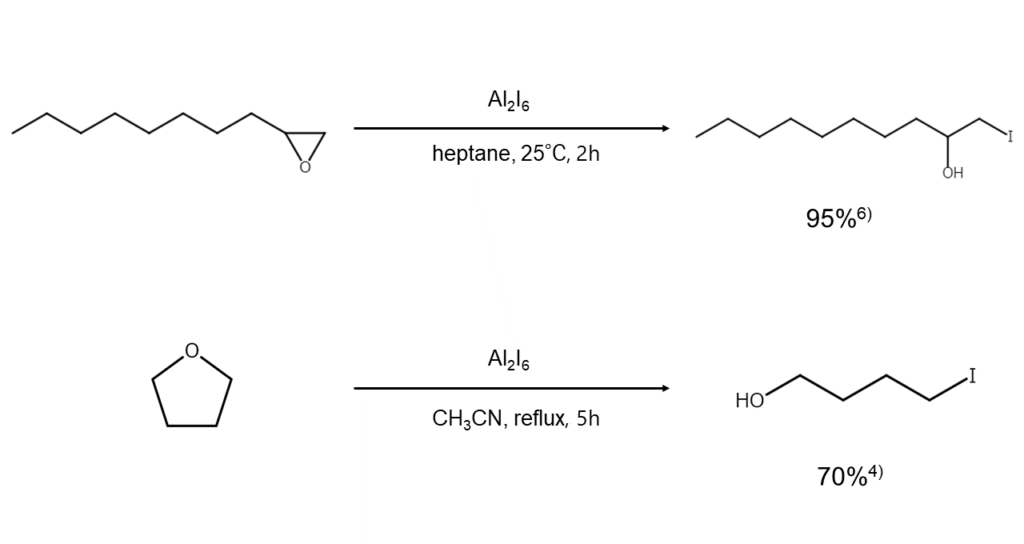
③ Ether and epoxide cleavage
Aluminum iodide can also be used in the cleavage of ethers and epoxides. It is known to have higher regioselectivity than reagents such as Al2Cl6 or Al2Br6, affording high yields of target products. The utility of aluminum iodide is even further enhanced when combined with a small amount of tetrabutylammonium iodide to significantly shorten reaction times.4),5)
Examples of ether cleavage using aluminum iodide are shown below.
Column: Aluminum iodide in semiconductor processes
We have taken a look at the basics of aluminum iodide as an iodinating agent in this article, but did you know that it is also used in the production of semiconductors?
Aluminum iodide is used to fabricate aluminum nitride (AIN) thin films. Manufacturing semiconductor products requires thin films, which can be made from one of many different materials, to be formed on silicon, sapphire, or other such substrate. One material used for thin films is AIN.
In recent years, many methods have been reported for forming high-quality AIN thin films. However, these methods frequently involve issues when considered for actual application, including fire risks and film-formation temperatures of over 1,000°C. There was a strong need for ways to form AIN thin films without the risk of fire and with only low film-formation temperatures.
A proposed solution to these issues is a film-formation method that uses aluminum iodide as a source material. A research team with Hiroki Iwane at Shizuoka University developed a technique to stably form thin films at low temperatures on sapphire substrates with aluminum iodide using chemical vapor deposition (CVD) under atmospheric pressure.7) The AIN thin film formed using this technique shows a substantial residual stress relaxation, giving favorable results.
Another application of aluminum iodide is in ion implantation procedures. Within the semiconductor manufacturing process, there is a procedure known as “ion implantation,” where impurity ions are introduced into silicon or another intrinsic semiconductor in order to change its properties.
Conventional methods of implanting aluminum ions into intrinsic semiconductors involve the generation of aluminum ions through the ionization of AIN or Al2O3 (alumina). However, US semiconductor-related company Axcelis Technologies has developed a method that produces aluminum ions from aluminum iodide.8) This method is said to be a major advancement for ion implantation systems.
While aluminum iodide is most widely known as a catalyst in the field of organic chemistry, the material is poised to make an incredible impact on the semiconductor field.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing. (Japanese)
2) Dutta, D. K., Lekhok, K. C. et al. Chem. Ind. (London), 1991, 175.
3) Sarmah, P., Barua, N. C. Tetrahedron, 1989, 45, 3569.
4) Bhatt, M. V., Babu, J. R. Tetrahedron Lett., 1984, 25, 3497.
5) Andersson, S. Synthesis, 1985, 437.
6) Eisch, J. J., Liu, Z. et al. J. Org. Chem., 1992, 57, 5140.
7) Iwane, H. et al. “Preparation of AlN thin films by means of CVD using iodide source under atmospheric pressure”, <https://www.jstage.jst.go.jp/article/pcersj/2008S/0/2008S_0_3C09/_article/-char/en> (Japanese)
8) Patent JP2020-522838 “Hydrogen cogas when aluminum iodide is used as the ion source material”.







