Bromination reactions with phosphorus bromides (bromo-phosphoranes): Phosphorus bromides (4): Discussion series on bromination/iodination reactions 42
In this series, we discuss MANAC’s specialties of bromination and iodination reactions. This article continues our exploration of bromination reactions with phosphorus bromides.
Our focus for this article is on reactions that use bromo-phosphoranes as reagents. We take a close look at two bromo-phosphorane reagents, dibromo(triphenyl)phosphorane and 1,2-bis(dibromo-diphenyl-phosphoranyl)ethane, and discuss common bromination reactions that utilize them.
Be sure to enjoy the article in its entirety as we cover must-see information related to the many advantages offered by these reagents in reactions for synthesizing bromoalkanes from alcohols, including low rates of byproducts and the ability to achieve bromoalkane conversions that maintain protected OH groups.
contents
Bromination reactions with phosphorus bromides: Reactions that use bromo-phosphoranes as reagents
Dibromo(triphenyl)phosphorane (bromine-triphenylphosphine)
Dibromo(triphenyl)phosphorane ((C6H5)3PBr2) is a pentavalent phosphorous compound that occurs when triphenylphosphine is treated with an equimolar amount of bromine, and is a colorless crystal that exhibits hygroscopicity. When exposed to humid air, it generates hydrogen bromide (HBr) and decomposes. Dibromo(triphenyl)phosphorane also readily decomposes when it comes into contact with oxidants and bases. The compound is highly soluble in dichloromethane, acetonitrile, benzonitrile, and N,N-dimethylformamide (DMF), and is slightly soluble in benzene and chlorobenzene.
Because it is corrosive and causes irritation to the skin and mucous membranes, dibromo(triphenyl)phosphorane should only be handled inside a fume hood. When storing, ensure that the compound is tightly sealed and placed in a cool, dark, and dry environment.2)
Bromine reactions (i) Synthesis of bromoalkanes from alcohols
Reaction mechanisms
In a polar solvent, dibromo(triphenyl)phosphorane exhibits behavior as a bromophosphonium salt ([(C6H5)3P+Br]Br–). Reacting this bromophosphonium salt with an alcohol will incur a rapid ligand exchange, changing it into an alkoxyphosphonium salt (Formula 1 below). It subsequently undergoes a nucleophilic attack from bromide ions, which causes the compound to gradually decompose into a bromoalkane and a phosphine oxide (Formula 2 below). The bimolecular nucleophilic substitution (SN2) reaction means that optically active alcohols yield bromoalkanes of inverted configurations.2)
Formula 1: [(C6H5)3P+Br]Br– + ROH → [(C6H5)3P+OR]Br– + HBr
Formula 2: [(C6H5)3P+OR]Br– → RBr + (C6H5)3P=O
These reactions are mostly used to synthesize bromoalkanes from primary and secondary alcohols. Since the reactions can be carried out with organic solvents (mainly acetonitrile or DMF) under neutral conditions, they are useful for substituting the hydroxy groups of acid- or base-sensitive sugars and nucleotides with bromine atoms.
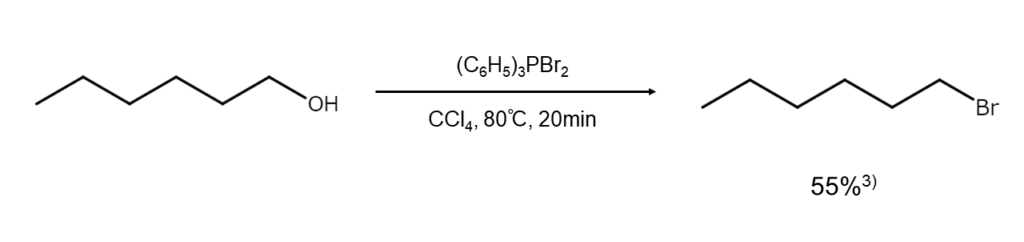
The following illustrates an example of the synthesis of a bromoalkane from an alcohol using dibromo(triphenyl)phosphorane.
Benefits include resistance to skeletal rearrangement
These reactions can be effectively applied to compounds containing cyclopropyl groups, olefin bonds, and other such highly reactive functional groups. Additionally, since these reaction processes do not generate carbocations, they do not readily cause skeletal rearrangements and offer a low rate of byproduct generation.
For example, neopentyl alcohol is especially prone to isomerization when HBr or phosphorus(III) bromides are used. However, using dibromo(triphenyl)phosphorane instead will convert neopentyl alcohol into a bromide without isomerization.4)
Methods with alternative bromine sources
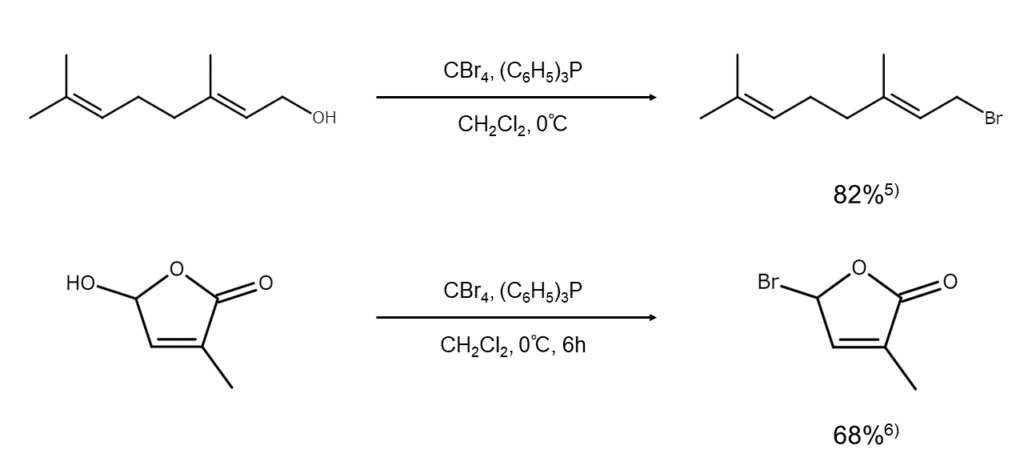
In certain cases where dibromo(triphenyl)phosphorane (a combination of elemental bromine and triphenylphosphine) fails to achieve satisfactory performance, excellent results may be achieved by using compounds such as N-bromosuccinimide (NBS) and carbon tetrachloride in place of elemental bromine. Here are some examples.
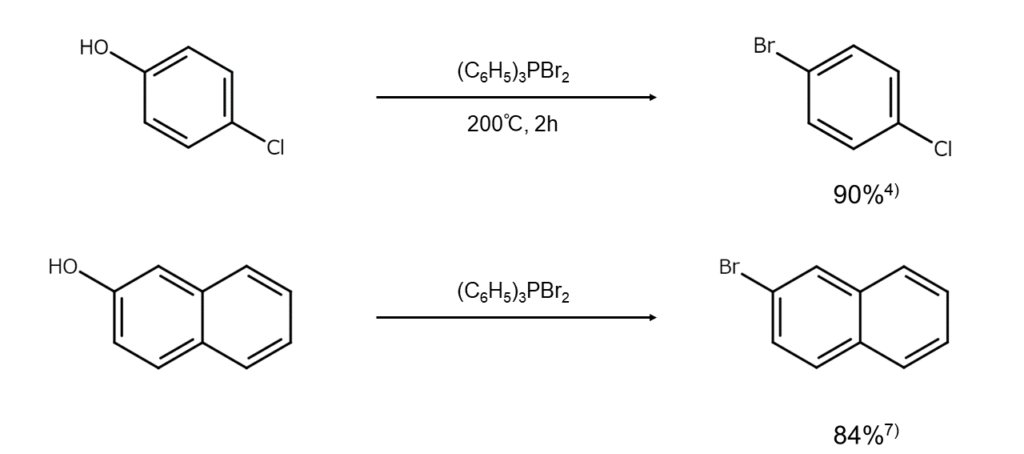
Bromine reactions (ii) Synthesis of bromoarenes from phenols
There are reports on methods that convert phenols into aryl bromides using dibromo(triphenyl)phosphorane, such as the following examples.
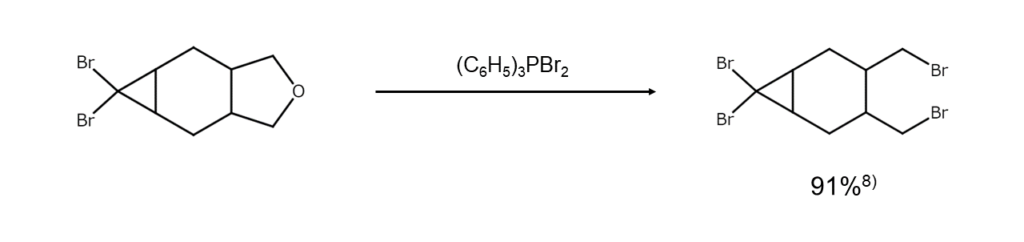
Bromine reactions (iii) Synthesis of bromoalkanes from ethers
Heating asymmetric dialkyl ether with dibromo(triphenyl)phosphorane will bring about a smooth brominative cleavage and yield a mixture of two different bromoalkanes. Chlorobenzene, benzonitrile, DMF, N-methyl-2-pyrrolidone (NMP), and other compounds with high boiling points are used as reaction solvents in these reactions. Since ethers with primary or secondary alkyl groups will react at relatively low temperatures, solvent-based side reactions can be avoided. This allows for good yields of target products, as shown in this example.
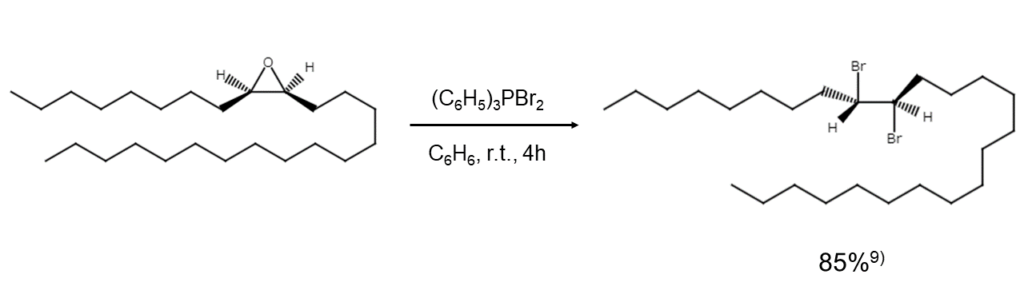
Using dibromo(triphenyl)phosphorane to open epoxide rings yields a vic-dibromoalkane. Although reactions with trans-epoxides lack stereoselectivity, resulting in isomer mixtures, reactions with cis-epoxides generate only erythro-dibromides, as shown below.9)
Bromoalkanes can be synthesized under moderate, neutral conditions when using trialkylsilyl ethers.4)
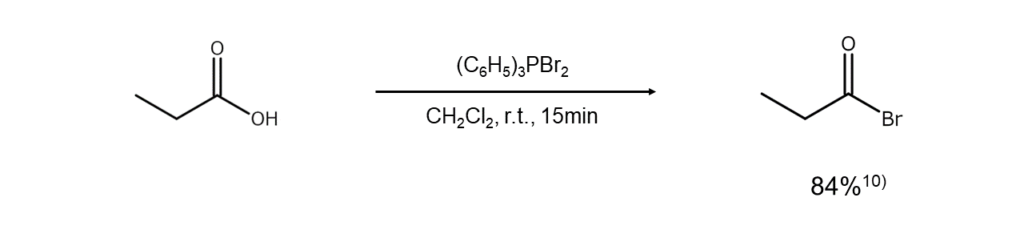
Bromine reactions (iv) Synthesis of acyl bromides from carboxylic acids, acid anhydrides, and esters
Acyl bromides can easily be obtained by reacting a carboxylic acid or an acid anhydride with dibromo(triphenyl)phosphorane.
1,2-Bis(dibromo-diphenyl-phosphoranyl)ethane
This bisphosphorane reagent ([(C6H5)2Br2P]CH2CH2[PBr2(C6H5)2]) is a useful compound capable of selectively brominating specific functional groups. It readily dissolves in chlorinated solvents, including the well-known dichloromethane, and stable storage of the solution can be achieved in a dark, cool environment. As with bromine, caution is required to avoid contact since 1,2-bis(dibromo-diphenyl-phosphoranyl)ethane is harmful to the skin and mucous membranes.
Bromination reactions
1,2-Bis(dibromo-diphenyl-phosphoranyl)ethane does not affect t-butyldimethylsilyl (TBDMS) groups or ester groups and will not react with the unsaturated bonds of alkenes or alkynes. The excellent functional group selectivity offers great utility when aiming to selectively brominate substructures of a polyfunctional compound.11)-13) For example, 1,2-bis(dibromo-diphenyl-phosphoranyl)ethane is used to convert polyfunctional alcohols into bromoalkanes under moderate conditions, as shown below.
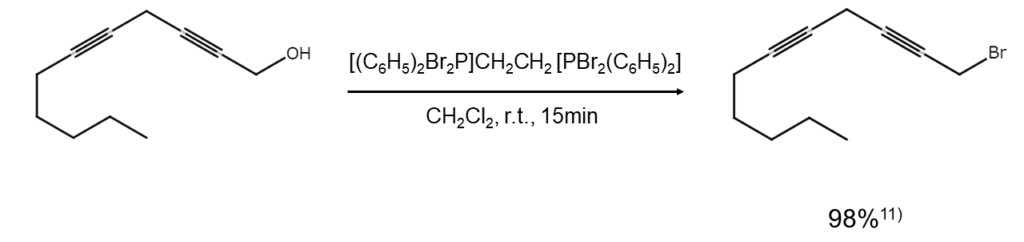
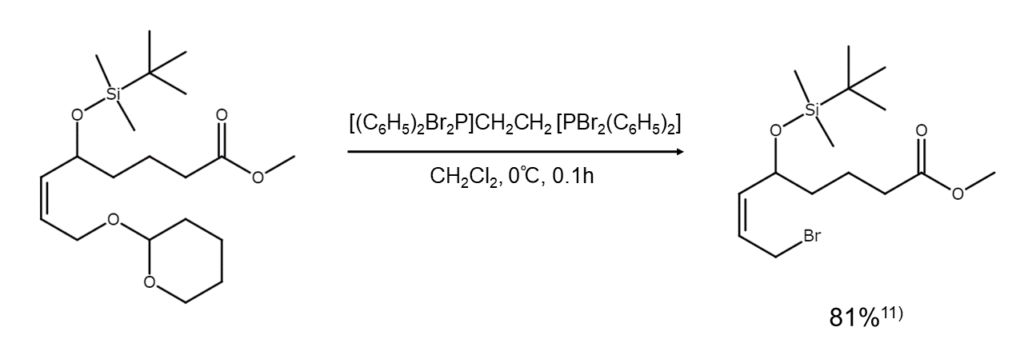
Further, using 1,2-bis(dibromo-diphenyl-phosphoranyl)ethane allows alcohols protected by tetrahydropyranyl groups to be converted directly into bromoalkanes.
Column: Reaction mechanism curveballs with DMF usage – The impacts of solvents on reaction mechanisms
So far in this article, we have explored how dibromo(triphenyl)phosphorane ((C6H5)3PBr2) behaves as a bromophosphonium salt ([(C6H5)3P+Br]Br–) in a polar solvent, and how this bromophosphonium salt constitutes a substantial starting material in reactions that use dibromo(triphenyl)phosphorane to synthesize bromoalkanes from alcohols. Let’s take another look at the reaction mechanism.
[(C6H5)3P+Br]Br– + ROH → [(C6H5)3P+OR]Br– + HBr
[(C6H5)3P+OR]Br– → RBr + (C6H5)3P=O
However, slight changes occur within these reactions when you use DMF ((CH3)2NCHO) as the solvent. As shown below, the bromoiminium salt ([BrCH=N+(CH3)2]Br–) generated in reactions between DMF and dibromophosphorane is believed to function as the actual reactive species.
(CH3)2NCHO + (C6H5)3PBr2 → [BrCH=N+(CH3)2]Br– + (C6H5)3P=O
[BrCH=N+(CH3)2]Br– +ROH → [ROCH=N+(CH3)2]Br– + HBr
[ROCH=N+(CH3)2]Br– → RBr + (CH3)2NCH(=O)
The take-home here is that, in some instances, a change in the solvent results in a corresponding change in the reaction mechanism.
References
1) MANAC Inc. Research Lab., Suzuki, H. (ed.). Shuuso oyobi Youso Kagoubutsu no Yuuki Gousei: Shiyaku to Gouseihou [Organic Syntheses of Bromine & Iodine Compounds: Reagents & Synthesis Methods], Maruzen Publishing (2017).
2) Schaefer, J. P., Weinberg, D. S. J. Org. Chem., 1965, 30, 2635.
3) Horner, L., Oediger, H. et al. Liebigs Ann. Chem., 1959, 626, 26.4) Wiley, G. A., Hershkowitz, R. L. et al. J. Am. Chem. Soc., 1964, 86, 964.
4) Kocienski, P. J., Cernigliaro, G. et al. J. Org. Chem., 1977, 42, 353.
5) Heather, J. B., Mittal, R. S. D. et al. J. Am. Chem. Soc., 1971, 98, 3661.
6) Schaefer, J. P., Higgins, J. J. Org. Chem., 1967, 32, 1607.
7) Kato, M., Nomura, S. et al. Chem. Lett., 1986, 281.
8) Sonnet, P. E., Oliver, J. E. J. Org. Chem., 1976, 41, 3279.
9) Alzpurua, J. M., Palomo, C. Synthesis, 1982, 684.
10) Schmidt, S. P., Brooks, D. W. Tetrahedron Lett., 1987, 28, 767.
11) Paquette, L. A., Rayner, C. M. et al. J. Am. Chem. Soc., 1990, 112, 4078.