
Iodination reactions with metalloid iodides: Discussion series on bromination/iodination reactions 28
Several articles in this series have covered iodination reactions that use metal iodides. This article wraps up our review of metal iodides by taking a look at iodination reactions with metalloid iodides.
Metalloid iodides are highly reactive, have a strong affinity for oxygen, and are used in synthesizing iodine compounds under mild conditions. In this article, we discuss iodination reactions that use iodosilanes, which are silicon iodides, and iodoboranes, which are boron iodides.
Be sure to read the entire article as we also discuss details of iodotrimethylsilane and boron triiodide, both commonly used in the laboratory.
contents
Iodosilane characteristics and iodination reactions
① Iodotrimethylsilane
Overview
Iodotrimethylsilane ((CH3)3SiI) is a mild Lewis acid reagent with a boiling point of 106°C and a density of 1.406 g/mL. It is used to remove protecting groups such as ethers, esters, acetals, and carbamates.
Although commercially available as a reagent, iodotrimethylsilane is relatively expensive. Consequently, in recent years, it is often used via in situ generation from chlorotrimethylsilane and NaI in acetonitrile.2) A method in which iodine is reacted with 3,6-bis(trimethylsilyl)-1,4-cyclohexadiene is also effective when generating a reagent in neutral conditions at a low temperature for immediate use.3)
However, caution is required when handling iodotrimethylsilane as it is sensitive to moisture and will cause severe skin irritation when contact occurs.
Iodine compound synthesis
High yields of iodoalkanes can be obtained by treating an alcohol with two equivalents of iodotrimethylsilane, making it practical for converting acid-sensitive alcohols into iodo-compounds.4)
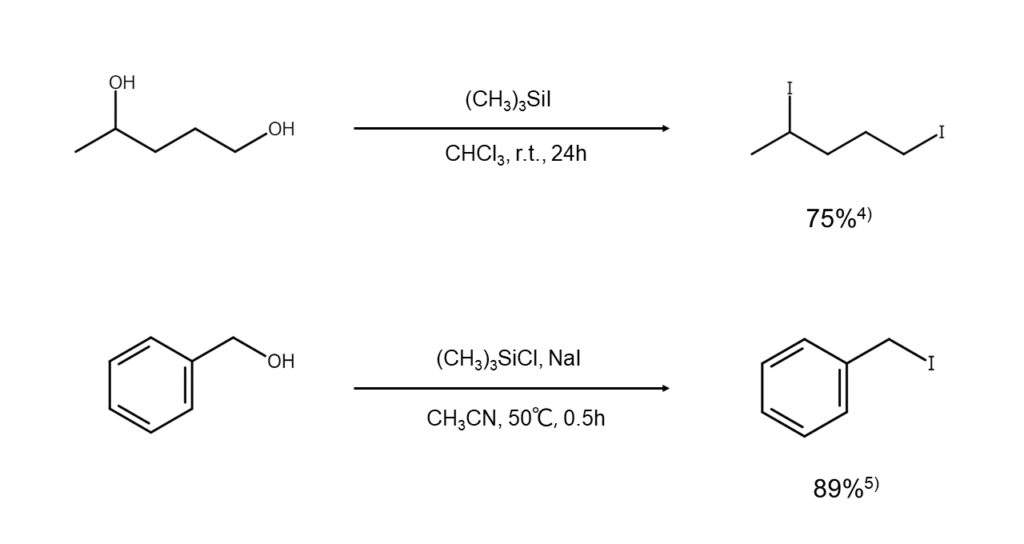
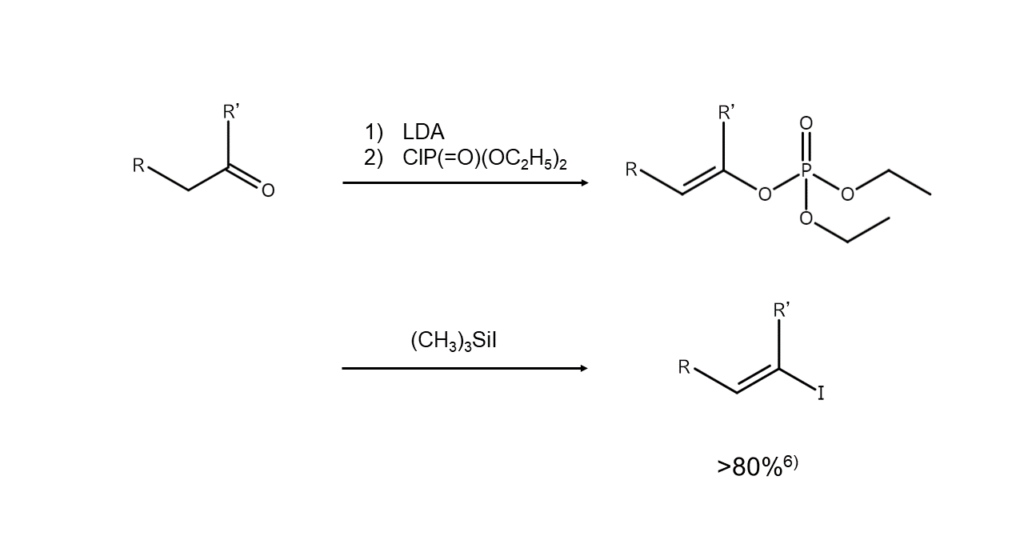
Further, 1-iodoalkenes can be obtained by first converting a ketone into an enol phosphate and reacting it with iodotrimethylsilane.6) The figure below shows the general method for this reaction.
Iodotrimethylsilane is also suitable for the hydroiodation of alkenes and the iodination cleavage of lactones, such as those shown below.
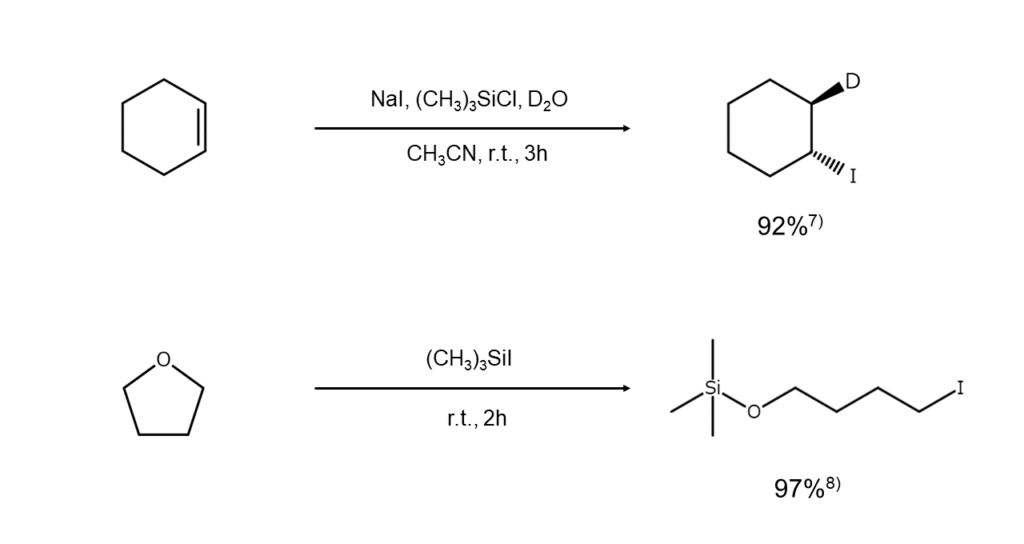
② Diiodosilane
Overview
Diiodosilane (SiH2I2) is a colorless liquid that is sensitive to moisture and has a melting point of -1°C, boiling point of 149.5°C (56–60°C/25 mmHg), a density of 2.7943 g/mL, and a flash point of 38°C. It is generally prepared by reacting phenylsilane with iodine at a low temperature but is also commercially available as a reagent.
Iodine compound synthesis (reagent comparison)
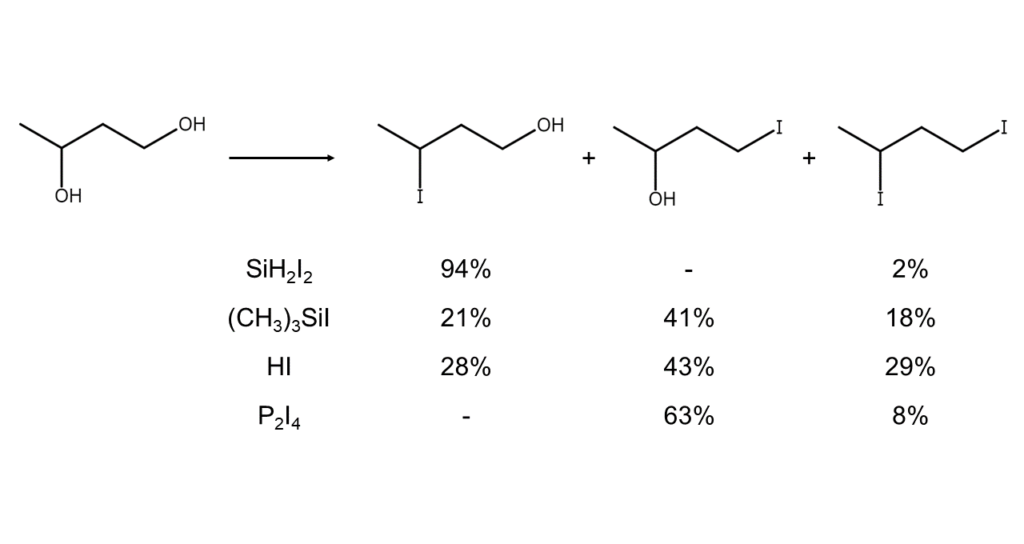
Like iodotrimethylsilane, diiodosilane can also be used to synthesize iodine compounds from alcohols. However, the two reagents have slightly different reactivities.
The reactivities of diiodosilane, iodotrimethylsilane, and HI in the iodination of alcohols are compared in Keinan’s research.9) The results demonstrate the differences in reactivity based on the reagent used, as shown below.
| Reagent | Reactivity |
| Diiodosilane | Secondary alcohols >> CH3OH > primary alcohols |
| Iodotrimethylsilane | CH3OH > primary alcohols > secondary alcohols |
| HI |
Based on these results, Keinan concluded the Lewis acidity of the silicon atoms in diiodosilane to be stronger than those in iodotrimethylsilane. Conversely, the nucleophilicity of the iodine atoms in iodotrimethylsilane was found to be greater than those in diiodosilane. This implies that reactions might occur via SN2 pathways when using iodotrimethylsilane, while reactions using diiodosilane may exhibit mechanisms of partial SN1 nature.
The table below compares the reactivities of diiodosilane, trimethylsilane, HI, and P2I4 toward primary and secondary alcohol groups.9)
③ Trichloroiodosilane
Overview
Trichloroiodosilane (SiCl3I) is another colorless liquid that is sensitive to moisture. It has a melting point of -60°C, a boiling point of 114.5°C, and is generated when treating SiCl4 with NaI or HI.
Iodine compound synthesis
Trichloroiodosilane cleaves ethers in the opposite direction of BBr3 and 9-Br-9-BBN, and is characterized by its higher regioselectivity over trimethyliodosilane.
Iodoborane characteristics and iodination reactions
① Boron triiodide
Overview
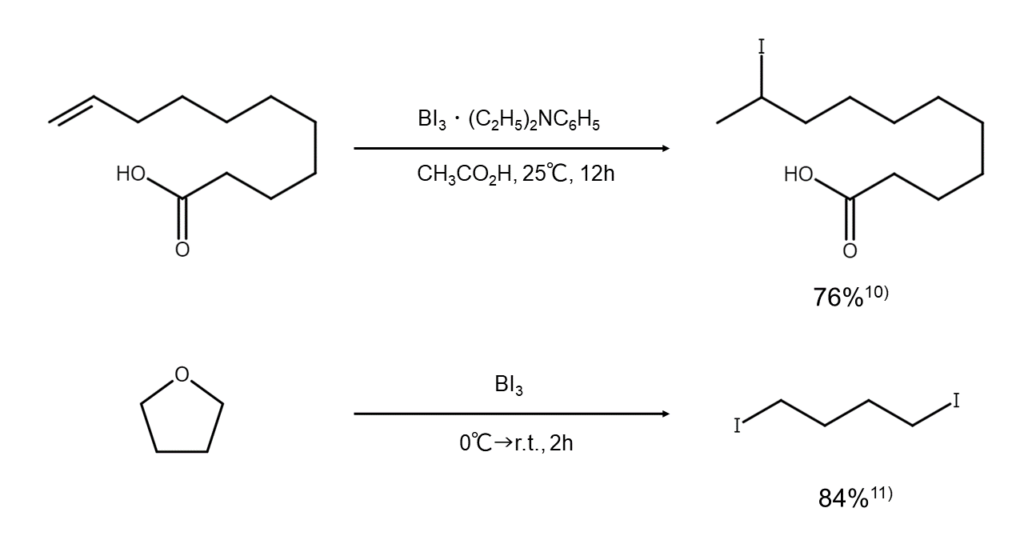
Boron triiodide (BI3) is a heavy liquid that is sensitive to moisture and has a density of 3.35 g/mL. Contact must be avoided as boron triiodide will cause severe irritation to skin and mucous membranes. Boron triiodide stabilizes when converted into an amine complex, making it easy to handle.
Iodine compound synthesis
Similar to BBr3, boron triiodide is used in the synthesis of iodoalkanes as it readily cleaves ether bonds and ester bonds at low temperatures. Reaction examples are shown below.
Column: The role of halosilanes in semiconductor manufacturing processes
Did you know that halosilanes, including the diiodosilane reviewed as an iodinating agent in this article, play an important role in semiconductor manufacturing processes?
Manufacturing semiconductors requires the formation of silicon-containing films on substrates using a method such as chemical vapor deposition (CVD) or atomic layer deposition (ALD). Halosilanes are used as raw materials for silicon-containing films.
Since halosilanes must be manufactured efficiently on an industrial scale in order to use them as film-forming materials, there have been systems devised in recent years for the large-scale, safe, and efficient manufacturing of diiodosilane (such as in Patent Publication JP2020-063176).
Nowadays, we are surrounded by products with integrated semiconductors, from computers to cellphones and even household appliances. Take a moment to think about halosilanes when using these devices.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing. (Japanese)
2) Olah, G. A., Narang, S. C. et al. J. Org. Chem., 1979, 44, 1247.
3) Jung, M. E., Blumenkopf, T. A Tetrahedron Lett., 1978, 3657.
4) Jung, M. E., Ornstein, P. L. Tetrahedron Lett., 1977, 2659.
5) Morita, T., Yoshida, S. et al. Synthesis, 1979, 379.
6) Lee, K., Wiemer, D. F. Tetrahedron Lett., 1993, 34, 2433.
7) Irifune, S., Kibayashi, T. et al. Synthesis, 1988, 366.
8) Poleschner, H., Heydenreich, M. et al. Synthesis, 1991, 1231.
9) Keinan, Pure Appl. Chem., 1989, 61, 1737.
10) Reddy, Ch. K., Periasamy M. Tetrahedron Lett., 1990, 31, 1919.
11) Povlock, T. P. Tetrahedron Lett., 1967, 4131.













