
Iodination of phenols, phenol ethers, anilines, and aniline-related compounds: Aromatic compound iodination reactions (2): Discussion series on bromination/iodination reactions 16
Elemental iodine exists as a solid at room temperature. Owing to its ease of handling and lower risks over other halogens, elemental iodine is commonly used as an iodinating agent. Continuing from where we started in the previous issue of this series, we are discussing aromatic compound iodination reactions with elemental iodine.
This article covers the iodination of aromatic compounds strongly activated by electron-donating groups, such as phenols and anilines.
Interestingly, active aromatic compounds are known to decompose more readily with elemental iodine. So how is iodination achieved with a compound that decomposes so easily? Here, we dive in to discuss details of reaction mechanisms and example reactions. Please be sure to use this information as a reference for experiments.
■ What you can learn from this article ✔ In the iodination of aromatic compounds with elemental iodine, only the substitution of hydrogen on the aromatic ring occurs; addition reactions or iodination of alkyl side chains do not take place. ✔ Highly active aromatic compounds are prone to oxidative decomposition by elemental iodine, making it necessary to convert elemental iodine into a different active species before iodination. ✔ Since isomers are generated during iodination, selectivity is crucial. MANAC’s patented technology enables the production of high-purity 3-iodoaniline. ■ Recommended Articles ・ Iodination of carboxylic acid, nitro compounds, and other inactive aromatic compounds: Aromatic compound iodination reactions (3): Discussion series on bromination/iodination reactions 17 ・ Hydrocarbon iodination: Aromatic compound iodination overview and reactions: Aromatic compound iodination reactions (1): Discussion series on bromination/iodination reactions 15
contents
Describing the iodination of aromatic compounds using elemental iodine
Significantly different from chlorination and bromination
The words “iodination of aromatic compounds” likely raise questions for some, such as “What parts of the compounds can be iodinated?” or “Do addition reactions occur in conjunction with the substitution reactions?”
However, when reacting an aromatic compound with elemental iodine, the only thing that can be achieved is the substitution of a ring hydrogen. Neither iodination of the alkyl side chain nor the addition of iodine to the aromatic ring is observed. This point makes the iodination of aromatic compounds vastly different from its bromination and chlorination counterparts.
There are also unique characteristics in terms of reaction conditions. Because no reaction occurs simply by mixing an aromatic compound with elemental iodine, an oxidizing agent or a strong acid catalyst is typically used to increase reactivity.
Aromatic compound iodination reactions with elemental iodine: Iodination of phenols and phenol ethers
Iodination through the generation of a different active species
Phenols are aromatic compounds strongly activated by a hydroxy group. Active aromatic compounds readily undergo oxidative decomposition with elemental iodine, so iodination requires elemental iodine to first be converted into a different active species.
Common methods first dissolve phenol in dilute NaOH aqueous solution, NaHCO3 or NaOCOCH3 aqueous solution, ammonia water, or ethylenediamine aqueous solution, and then bring about iodination using hypoiodous acid (HOI) (see the formula below) generated in situ from elemental iodine.
I2 + H2O ⇌ HOI + H+ + I–
There is also a known method using a morpholine-iodine complex as an iodinating agent under the same mild basic conditions. In this method, iodination is driven by phenol directly attacking the iodine atoms in the morpholine-iodine complex.
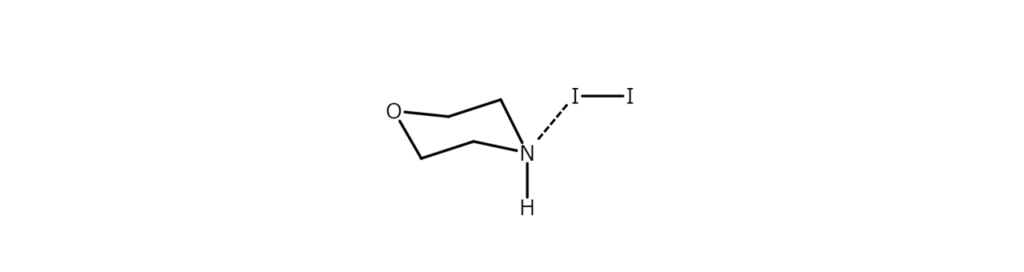
When the active species above is set to act on phenol, iodination occurs smoothly, starting at the para position with its small steric hindrance, and then advancing to the ortho position. Iodination of the meta position does not occur. High ortho orientation may be observed when using a transition metal salt or NaNO2 as an auxiliary agent to coordinate metal ions to the oxygen atoms of phenol.
Reaction precautions
Phenol ethers and phenols with a deactivating group (such as a carboxy group or a nitro group) have a lower reactivity than other phenols. For this reason, cases in which an oxidizing agent is combined to iodinate these compounds are not uncommon.
Also, as phenols with a carboxy group at the activation position readily decarboxylate during iodination, caution should be exercised regarding reaction conditions.
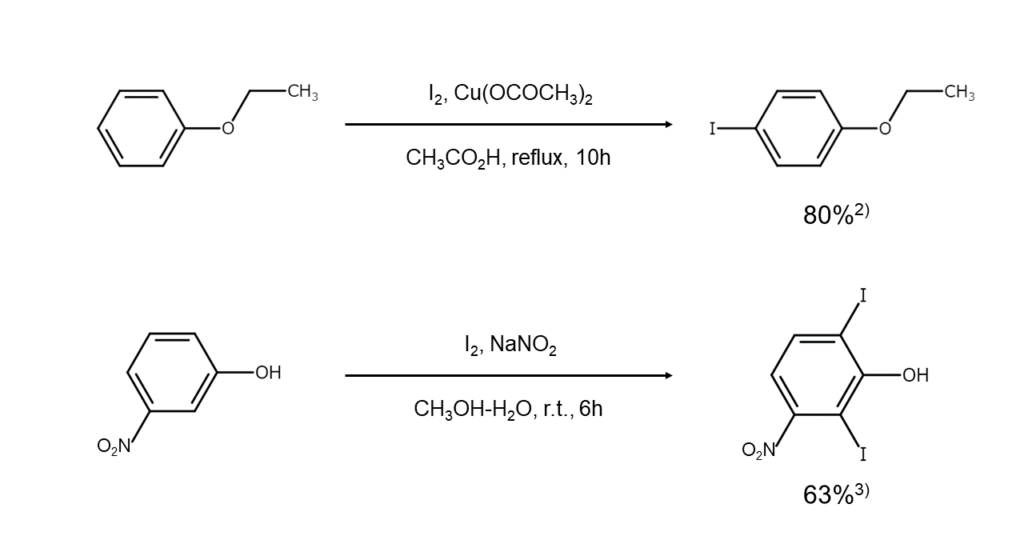
Reaction examples
Iodination examples for anilines and related compounds are shown below.
Aromatic compound iodination reactions with elemental iodine: Iodination of anilines and related compounds
As with phenols, anilines iodinate with a different active species
Like phenols, anilines are strongly activated aromatic compounds. Hence, the iodination reaction outline for anilines is essentially identical to that of phenols.
Aromatic amines, such as anilines and other related compounds, have properties that cause them to easily change into dendritic materials through the action of elemental iodine. Therefore, iodination generally occurs with hypoiodous acid formed in situ from elemental iodine, or with a morpholine-iodine complex. Iodination occurs first at the para position and subsequently at the ortho position. There is no iodination at the meta position.
Reaction precautions
Reaction precautions are also closely similar to those of phenols.
Oxidizing agents are frequently required in order to iodinate deactivated amines. Additionally, amines with a carboxy group at the activation position require caution as they readily decarboxylate during iodination.
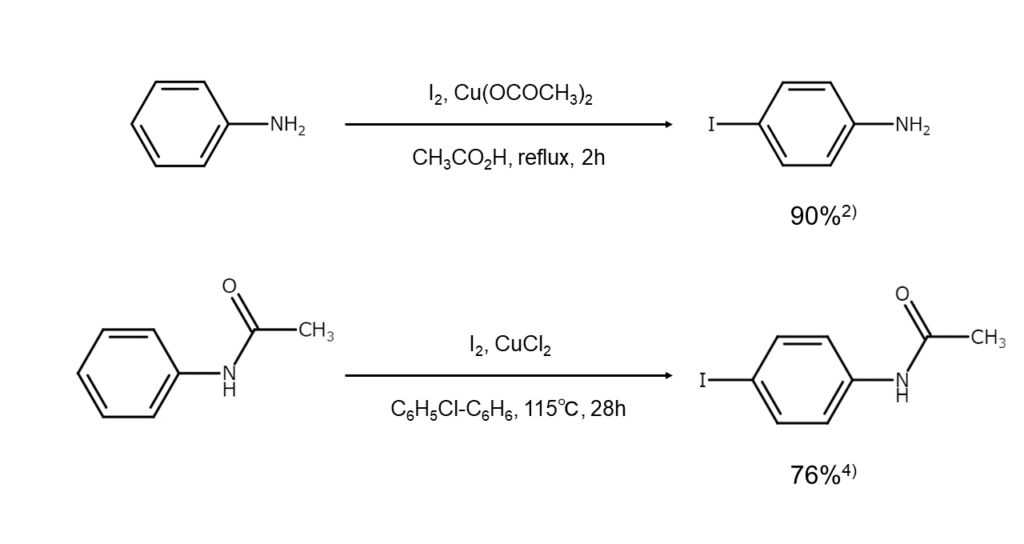
Reaction examples
Iodination examples for anilines and related compounds are shown below.
MANAC Original Technology: What does it take to selectively extract 3-iodoaniline?
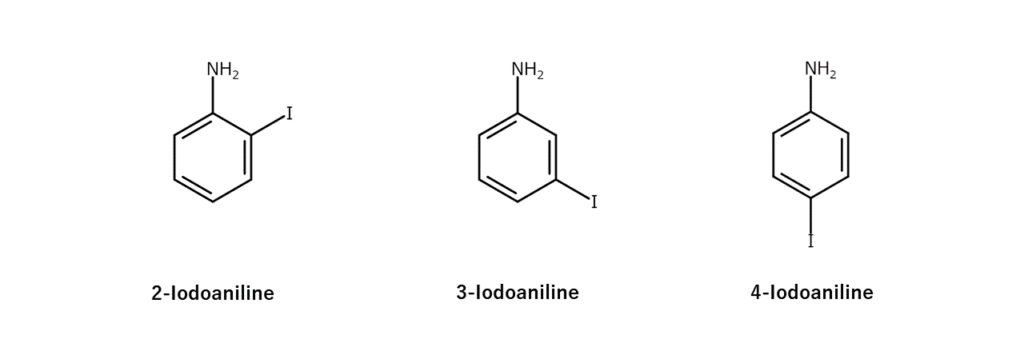
Achieving the iodination of an aromatic compound does not guarantee an efficient yield of the target compound. What stands in the way is the issue of isomers. Let’s look at an example.
3-Iodoaniline, which is an aniline iodinated at the 3-position carbon, is a valuable compound as an intermediate in pharmaceuticals and electronic materials. However, synthesizing 3-iodoaniline through general methods will also generate 2-iodoaniline and 4-iodoaniline isomers on the order of several percent. This drawback necessitated a technique to selectively extract only the target 3-iodoaniline.
The solution to this problem came from MANAC’s patented technologies. By treating iodoaniline isomer mixtures with a “certain process,” MANAC successfully obtained 3-iodoaniline at a high purity of over 99%.
What kind of method did MANAC use to purify 3-iodoaniline, you ask? To find out more, be sure to check the article below.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing. (Japanese)
2) Horiuchi, C. A., Satoh, J. Y. Bull. Soc. Chem. Jpn., 1984, 57, 2691.
3) Kiran, Y. B., Konakahara, T. et al. Synthesis, 2008, 2327.
4) Baird, Jr. W. C., Surridge, J. H. J. Org. Chem., 1970, 35, 3436.








