
Hydrocarbon iodination: Aromatic compound iodination overview and reactions: Aromatic compound iodination reactions (1): Discussion series on bromination/iodination reactions 15
In this series, we discuss bromination and iodination reactions, specialties of MANAC. Through the last issue, we closely reviewed iodination reactions that use a halogen exchange. Iodides have applications in a wide range of fields and many synthesis methods. Starting with this issue, we will cover one of these methods for iodide synthesis: the iodination of aromatic compounds using elemental iodine.
This article first touches upon the characteristics of elemental iodine, which lies at the heart of these reactions. We then detail aromatic compound iodination reactions that use elemental iodine. It is our hope that this article serves as reference material to help achieve a deeper understanding of aromatic compound iodination.
■ What you can learn from this article ✔ Iodine has bactericidal properties and is used in various fields such as iodine tincture, Lugol’s solution, catalysts, hygiene products, food and livestock feed additives, and pharmaceuticals. ✔ The iodination method of heating aromatic hydrocarbons with iodine under reflux in dilute nitric acid is frequently used industrially due to its simple operation and low-cost reagents. ✔ MANAC is not only skilled in bromination and iodination to create heterohalogen compounds, but also in various coupling reactions using heterohalogen compounds. ■ Recommended Articles ・ Acyl iodide synthesis, iodoarene synthesis: Iodination reactions with halogen exchange (3): Discussion series on bromination/iodination reactions 14 ・ Iodination of phenols, phenol ethers, anilines, and aniline-related compounds: Aromatic compound iodination reactions (2): Discussion series on bromination/iodination reactions 16 ・ Patent: Selectively manufacture needed compounds!
contents
Describing elemental iodine
Iodine, an essential chemical element for iodination reactions, has long been used in iodine tincture and Lugol’s solution thanks to its bactericidal properties. It also plays a vital role as synthetic raw material for photosensitive materials. Other uses of iodine span many fields, with applications in catalysts, polymer stabilizers, hygiene products, food and livestock feed additives, and pharmaceuticals.
While iodine is widely used in many different areas, its high cost makes collection and reuse highly desirable. The iodine waste generated from chemical reactions often occurs in the form of hydrogen iodide or alkali iodide. Elemental iodine, therefore, is collected through methods that oxidize such iodine waste with hydrogen peroxide, iodate, persulfate, or manganese dioxide.
Since elemental iodine is solid, it is also easier to handle than elemental bromine, which is a liquid.
Elemental iodine handling precautions
With certain qualities, such as low vapor pressure, iodine is safer to handle than other halogen elements. Regardless, as iodine is highly corrosive and is a severe irritant for eyes, mucous membranes in the upper respiratory tract, and skin, care must be taken to prevent contact and vapor inhalation.
Furthermore, long-term exposure to elemental iodine or other iodides can lead to skin sensitivity, even at low concentration levels. Caution is required as allergic reactions to iodine-based contrast agents used for diagnostic radiography may present as urticaria of the skin or mucous membranes, breathing difficulty, or other such symptoms.
Describing the iodination of aromatic compounds with elemental iodine
The iodination of aromatic compounds with elemental iodine occurs as a reaction in which the hydrogen present on an aromatic ring is substituted with iodine.
When chlorinating or brominating an aromatic compound, other reactions occur in addition to this hydrogen substitution reaction, such as bromination of the alkyl side chain of the aromatic ring and chlorine addition to the aromatic ring. However, these additional reactions are not seen with iodine. Such points make the iodination of aromatic compounds vastly different from its chlorination or bromination counterpart.
Reaction types
Several types of aromatic compound iodination reactions use elemental iodine, such as those listed below. Reaction details are given in the latter half of this article, with more reaction details planned for the next and other upcoming articles.
A) Iodination of aromatic hydrocarbons
Mixing aromatic hydrocarbons with iodine only generates pi-complexes and leads to no further reaction. Therefore, in order to raise the reactivity of iodine in a reaction, it is generally combined with an oxidizing agent or a strong acid catalyst.
B) Iodination of phenols and phenol ethers
One of the characteristics of active aromatic compounds such as phenols is that they easily undergo oxidative decomposition by the action of elemental iodine. This means that iodination occurs with the hypoiodous acid generated in situ from iodine under mild basic conditions. However, an oxidizing agent may be necessary to iodinate phenols or phenol ethers deactivated by other substituents.
C) Iodination of anilines and related compounds
Like phenols (reaction B above), the iodination of anilines and related compounds occurs with hypoiodous acid under mild basic conditions.
D) Iodination of carboxylic acid, nitro compounds, and other deactivated aromatic compounds
Iodination of aromatic compounds with low reactivity, such as carboxylic acid and nitro compounds, is carried out by combining iodine with a silver salt or oleum to form an iodine cationic species.
The above reactions require an auxiliary agent, such as an oxidizing agent or an acid catalyst. As shown in the table below, different combinations of aromatic hydrocarbon substrates and auxiliary agents result in significantly different yields.
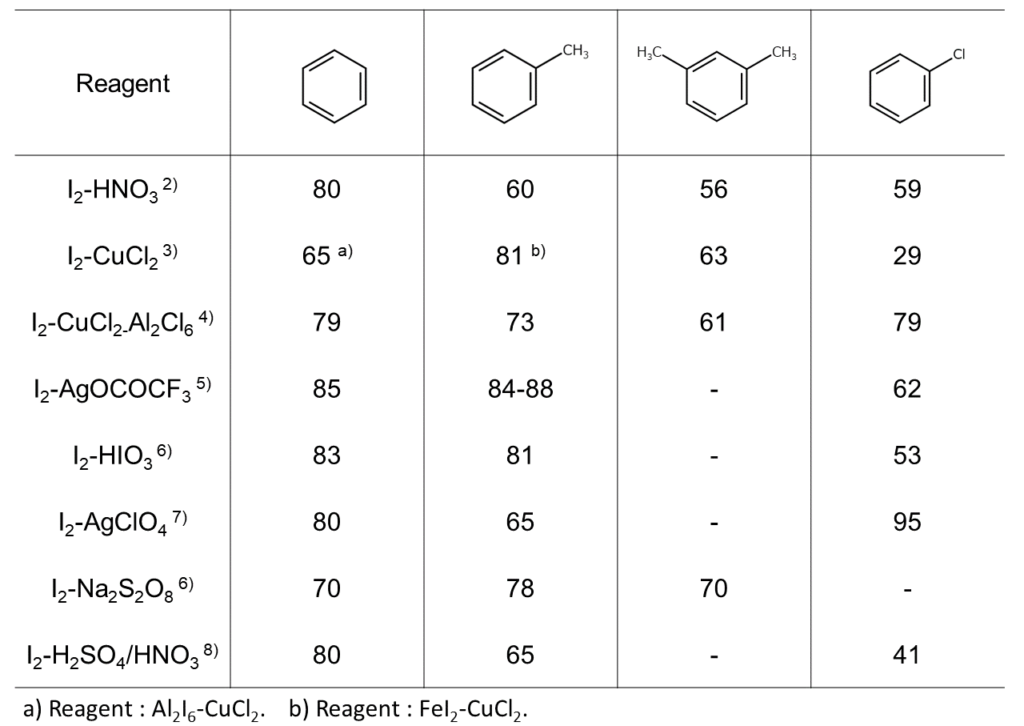
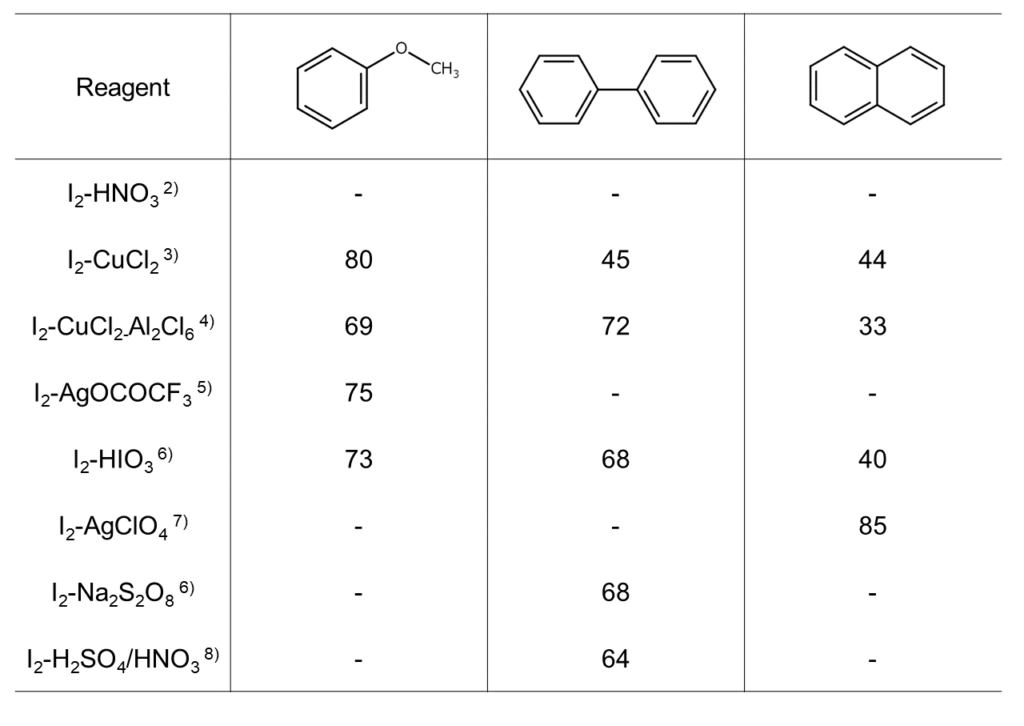
Aromatic compound iodination reactions with elemental iodine: Hydrocarbon iodination
Reaction details
In this article, we are looking into reactions that iodinate aromatic hydrocarbons using elemental iodine. Setting iodine to act on an aromatic hydrocarbon only results in the formation of colored pi-complexes and does not lead to any further reactions. For this reason, in order to iodinate aromatic rings, an oxidizing agent or a strong acid catalyst is used as an auxiliary agent.
Examples of auxiliary agents
The agents listed below are examples of auxiliary agents often used for these reactions.
- Iodic acid
- Periodic acid
- Dilute nitric acid
- Silver trifluoroacetate
- Ceric ammonium nitrate (CAN)/Ammonium cerium(IV) nitrate
In addition, acetic acid, water, or a mixed solution are often used as reaction solvents. However, when there are substrate solubility issues, a two-phase reaction system is also employed, which then includes the usage of dichloromethane, chloroform, or an ether.
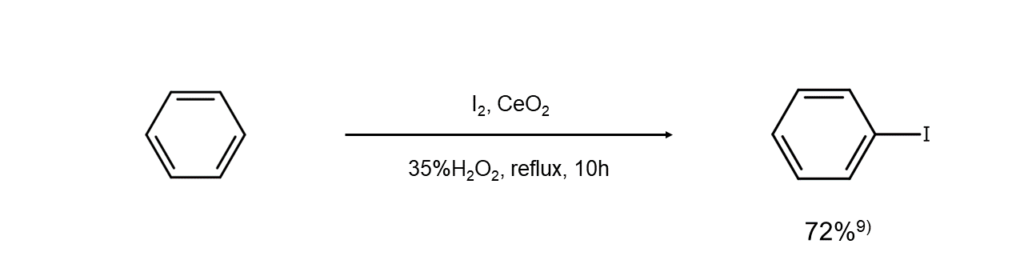
Examples of iodination reactions using a benzene or a biphenyl as a substrate are shown below.
Iodination of benzene
Iodination of biphenyl

Various reactions and precautions related to combinations with auxiliary agents or solvents
As we just touched on, auxiliary agents are required for the iodination of aromatic hydrocarbons with elemental iodine. These reactions differ depending on the auxiliary agent or solvent used, and impurities or harmful substances can form with certain combinations. The following gives various reactions and precautions for particular auxiliary agent and solvent combinations. Be sure to use this information as a reference.
– Easy management and low-cost reagents for reactions with dilute nitric acid as an auxiliary agent
Iodination methods that heat aromatic hydrocarbons with iodine in dilute nitric acid under a reflux are frequently used industrially as these methods afford easy management and low-cost reagents. Similar results can be obtained by heating these in nitrate dissolved in dilute sulfuric acid. However, caution is needed when using an alkylbenzene or a condensed polycyclic hydrocarbon, as nitro compound or oxide impurities will easily form.
– Reactions with heavy metal compounds useful for generating HOI and other iodinating agents
Combining a heavy metal compound, such as silver trifluoroacetate (AgOCOCF3) or mercury (II) oxide (HgO), with iodine under mild conditions is useful for generating iodinating agents, including HOI and CF3CO2I. In addition, when using this combination in an alcohol, an ester of hypoiodous acid (ROI) first forms. This decomposes and generates an alkoxy radical (RO•), and since this extracts hydrogen atoms from organic molecules, intramolecular cyclization and beta-fragmentation readily occur. This combination is also used for the alkoxyiodination of alkenes and the iodolactonization of unsaturated carboxylic acids. However, a large amount of metal iodides is generated during these reactions, which presents a severe drawback.
– Caution required for reactions using mercury salts
When iodine and a mercury salt are combined to iodinate an active aromatic compound, care must be taken as a large amount of harmful aryl mercury is generated.
Aromatic hydrocarbon iodination applications for heterohalogen compound synthesis
This article has covered the iodination of aromatic hydrocarbons using elemental iodine.
Applications of this aromatic hydrocarbon iodination include synthesizing heterohalogen compounds containing bromine (Br) and iodine (I), compounds that are a particular specialty of MANAC.
There are many different types of heterohalogen compounds, which are mainly used as intermediates (parts) in products such as medicines and photoconductive materials.
We discuss heterohalogen compounds in the following article: “Making complex compounds a breeze! The heterohalogen series”.
MANAC specializes not only in bromination and iodination to make heterohalogen compounds, but also in coupling reactions of various types using heterohalogen compounds. This enables MANAC to handle complete orders, from manufacturing intermediates to producing higher-order compounds. Please feel free to inquire.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing. (Japanese)
2) Datta, R. L., Chatterjee, N. R. J. Am. Chem. Soc., 1917, 39, 435; 1919, 41, 292.
3) Baird, Jr W. C., Surridge, J. H. J. Org. Chem., 1970, 35, 3436.
4) Sugita, T., Idei, M. et al. Chem. Lett., 1982, 1481.
5) a) Henne, A. L., Zimmer, W. F. J. Am. Chem. Soc., 1951, 73, 1362. b) Haszeldine, R. N., Sharpe, A. G. J. Chem. Soc. 1952, 993.
6) Wirth, H. O., Königstein, O. et al. Liebigs Ann. Chem., 1960, 634, 84.
7) Birckenbach, L., Goubeau, J. Chem. Ber., 1932, 65, 395.
8) Tronov, B. V., Novikof, A. N. Izv. Vyssh. Uschech. Zaved., Khim. Khim. Technol., 1960, 3, 872; Chem. Abstr., 1961, 55, 8348.
9) Peng Zhang et al. Adv. Synth. Catal., 2012, 354, 720.
10) Varma, P.S., Krishnamurti, M. J. Indian Chem. Soc., 1937, 14, 156.









