
Iodination of carboxylic acid, nitro compounds, and other inactive aromatic compounds: Aromatic compound iodination reactions (3): Discussion series on bromination/iodination reactions 17
In this series, we discuss bromination and iodination reactions, specialties of MANAC. So far, we have discussed in our two previous articles the iodination of aromatic hydrocarbons, phenols, and anilines as examples of aromatic compound iodination reactions using elemental iodine. In the last article, we covered the iodination of aromatic compounds strongly activated by electron-donating groups. For this article, we take on a different angle to discuss the iodination of nitro compounds, aromatic carboxylic acid, and other aromatic compounds deactivated by nitro groups, carboxy groups, and other electron-withdrawing groups.
As we previously discussed, because elemental iodine exists as a solid at room temperature, it has several advantages, including being safer and easier to handle than other halogens.
But regardless of its conveniences, some may doubt whether elemental iodine can iodinate inactive aromatic compounds. It is indeed difficult to iodinate inactive aromatic compounds using typical iodinating agents. However, using catalysts, auxiliary agents, or oxidants enables the iodination of inactive aromatic compounds. This article gives detailed examples of inactive aromatic compound iodination reactions, which can be used as a reference for experiments.
■ What you can learn from this article ✔ Special methods, such as using inorganic salts, fuming sulfuric acid, oxidizing agents, or fluorine, are required to iodinate compounds that do not react with standard iodinating agents. ✔ Selective iodination techniques enable the efficient production of target compounds with high yields, making them widely utilized in industrial and medical fields. ✔ In the body, tyrosine undergoes iodination during the synthesis of thyroid hormones, playing a crucial role in sustaining life through mechanisms similar to chemical reactions. ■ Recommended Articles ・ Alkane iodination: Aliphatic compound iodination overview and reactions: Aliphatic compound iodination reactions (1): Discussion series on bromination/iodination reactions 18 ・ Iodination of phenols, phenol ethers, anilines, and aniline-related compounds: Aromatic compound iodination reactions (2): Discussion series on bromination/iodination reactions 16 ・ Hydrocarbon iodination: Aromatic compound iodination overview and reactions: Aromatic compound iodination reactions (1): Discussion series on bromination/iodination reactions 15
contents
Aromatic compound iodination reactions with elemental iodine: Iodination of carboxylic acid, nitro compounds, and other inactive aromatic compounds
As we just mentioned, aromatics such as nitro compounds, carboxylic acids, nitriles, polyhalogen derivatives, and quinones have low reactivity, which makes iodination with standard iodinating agents difficult. The following kinds of methods are used to overcome this hurdle.
1) Generating hypoiodous acid or iodine cations using an inorganic salt
2) Generating iodine cations using oleum
3) Using a strong oxidant
4) Using fluorine
Reaction examples using these methods are introduced below.
1) Generating hypoiodous acid or iodine cations using an inorganic salt
The first method uses inorganic salt. Silver, mercury, copper, or other inorganic salts are used to generate hypoiodous acid or iodine cations from elemental iodine to achieve higher iodinating capacities, as is shown in the example below.
Ag2SO4 + 2I2 → 2I+ + SO42- + 2AgI
The resulting iodine cation attacks the aromatic ring and bonds to it by replacing the hydrogen.
Further, the iodination reaction with a silver salt, as used by Waters and others, is a famous reaction that dates back many years and is often used in laboratories for small-scale synthesis2.
Examples of inactive aromatic compound iodination reactions using iodine and a silver salt are shown below.
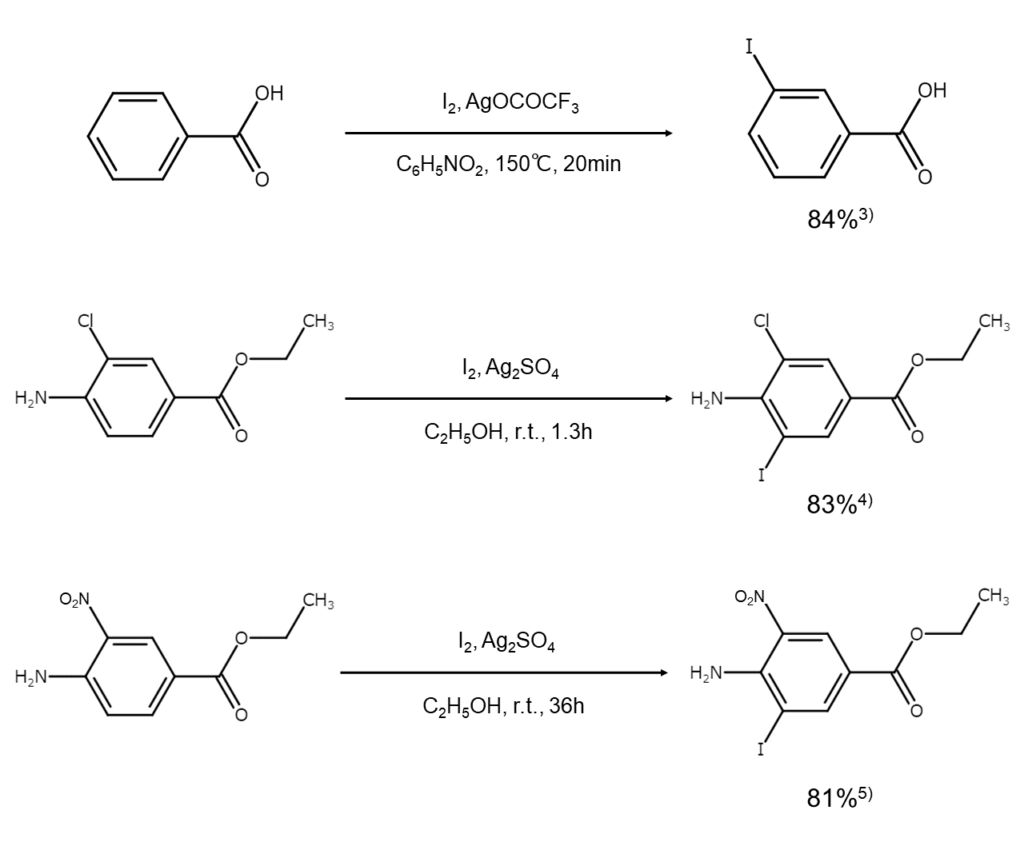
2) Generating iodine cations using oleum
The next method uses oleum. Typically, iodine mostly does not dissolve in sulfuric acid. However, iodine will dissolve in oleum and change into a higher-order iodine cation (I3+, I5+, I7+) in the solution. Adding an inactive aromatic compound to the solution and applying heat will give rise to the polyiodination of the aromatic ring. Using this method allows for even the hexaiodination of benzene, something not possible with typical methods.

3) Using a strong oxidant
In addition to the aromatic ring polyiodination with oleum as described above, there is also a method to use potassium persulfate (K2S2O8) as an oxidant to generate iodine cations in order to polyiodinate inactive aromatic compounds.

4) Using fluorine
The last method in this introduction involves fluorine as an auxiliary agent and is the most potent way to iodinate inactive aromatic compounds using elemental iodine.
First, the substrate-to-iodine mole ratio is selected based on the target number of iodine atoms to be introduced. For example, a substrate-to-iodine mole ratio of 1:2.2 is selected when targeting the introduction of two iodine atoms, and a mole ratio of 1:3.2 is selected when targeting the introduction of three iodine atoms. These are dispersed in a mixed solution of 98% sulfuric acid and Freon 113 (CCl2FCCl2F), and while stirring at room temperature, fluorine gas diluted to 5–10% in nitrogen is bubbled into the solution. Since the iodination then rapidly completes, the reaction mixture is poured into ice water, and the reaction product is extracted with dichloromethane. Removing the solvent and purifying the results by distilling or recrystallizing the residue enables polyiodine compounds to be obtained.
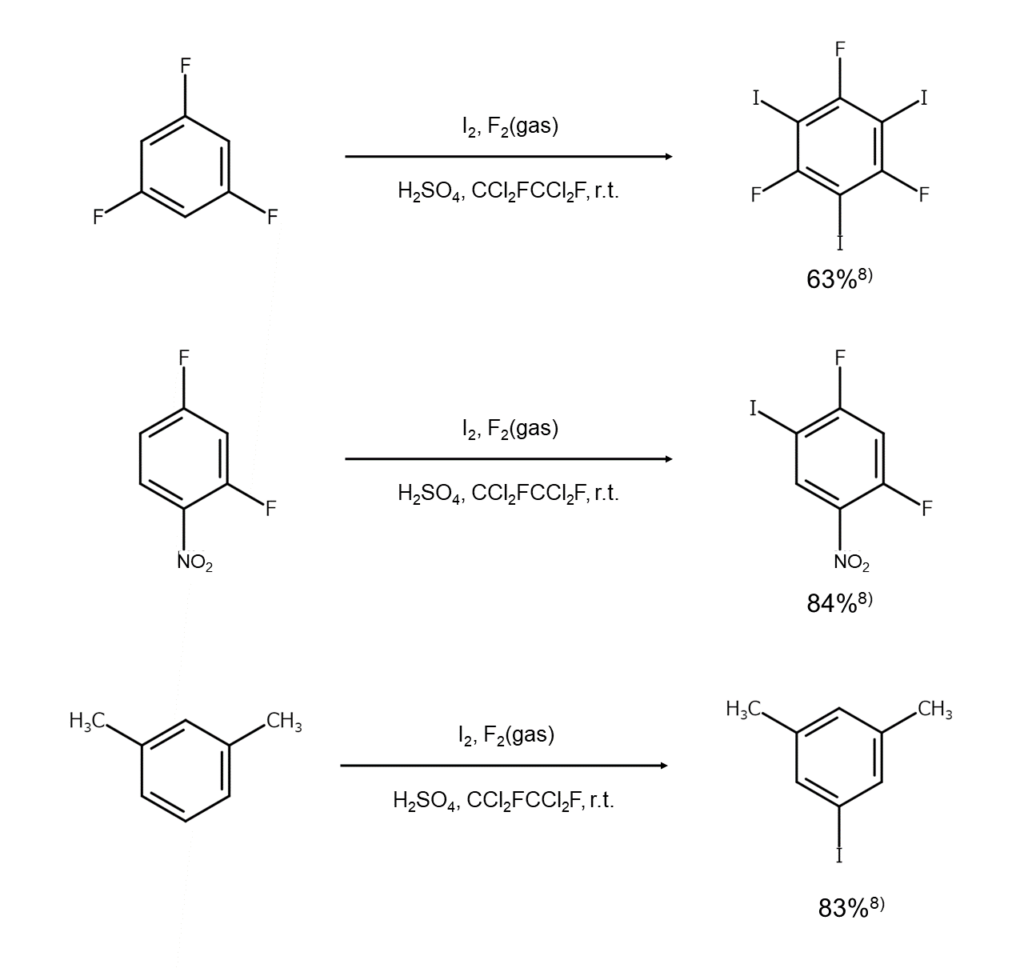
Being able to selectively obtain targeted polyiodine compounds by adjusting the substrate-to-iodine mole ratio and the ability to quickly gain such targeted compounds at a high yield are some of the major advantages of this method.
As illustrated, these methods discussed above make iodination possible even for the seemingly challenging iodination of inactive aromatic compounds. Perhaps some wonder, “We want to make this compound, but can it be done?” It may just be possible with MANAC’s technologies for iodination and iodine management. Be sure to inquire with MANAC.
The Breakroom Column:
Did you know that aromatic compound iodination occurs inside our bodies?
So far, our discussion of the iodination of aromatic compounds with elemental iodine has spanned three articles. Since simply mixing elemental iodine with an aromatic compound does not cause a reaction, the help of an auxiliary agent or catalyst is enlisted in line with the type of aromatic compound, which acts as the substrate. We have explained this as the defining aspect of aromatic compound iodination with elemental iodine.
Such aromatic compound iodination with elemental iodine occurs not only in the laboratory but also within our bodies. This happens in the secretion process of thyroid hormones triiodothyronine and thyroxine.
Thyroid hormones are involved in the developmental and metabolic functions of the body. Iodine and tyrosine (an aromatic alpha-amino acid) are particularly important as two raw materials for synthesizing these thyroid hormones. During the secretion process for thyroid hormones, iodination of the aromatic compound tyrosine occurs, and triiodothyronine (T3), which has three iodine atoms attached, and thyroxine (T4), which has four iodine atoms, are generated. What kind of mechanisms bring about these reactions? Let’s dive into the secrets behind them.
When the thyroid gland is stimulated by commands it receives from the pituitary gland in the brain, the synthesis of thyroid hormones begins inside the follicular epithelial cells located within the thyroid. The thyroid contains hundreds of colloid-filled thyroid follicles. The interior surface of these follicles is lined with thyroid follicular epithelial cells. Further inside the follicular lumen is where you will find the site of thyroid hormone synthesis.
The thyroid hormone synthesis process can be divided into the three major stages below.
Thyroid hormone synthesis process
- Iodine collection
- Tyrosine iodination
- Triiodothyronine and thyroxine generation through coupling
Iodine collection
The iodine in food is absorbed when eaten and taken into the blood. When the thyroid is stimulated by commands from the brain, the thyroid follicular epithelial cells collect iodine from blood vessels. The collected iodine passes through the thyroid follicular epithelial cells and is released into the follicular lumen.
Tyrosine iodination
The substrate thyroglobulin and the oxidase enzyme known as peroxidase are synthesized within the thyroid follicular epithelial cells and released into the follicular lumen. Thyroglobulin contains a large number of tyrosine residues (aromatic alpha-amino acid). It is then through the action of peroxidase, also synthesized by the follicular epithelial cells, that iodine is oxidized and changed into an iodine cation. This iodine cation acts on the aromatic tyrosine contained in thyroglobulin. The tyrosine is then iodinated and converted into monoiodotyrosine and diiodotyrosine.
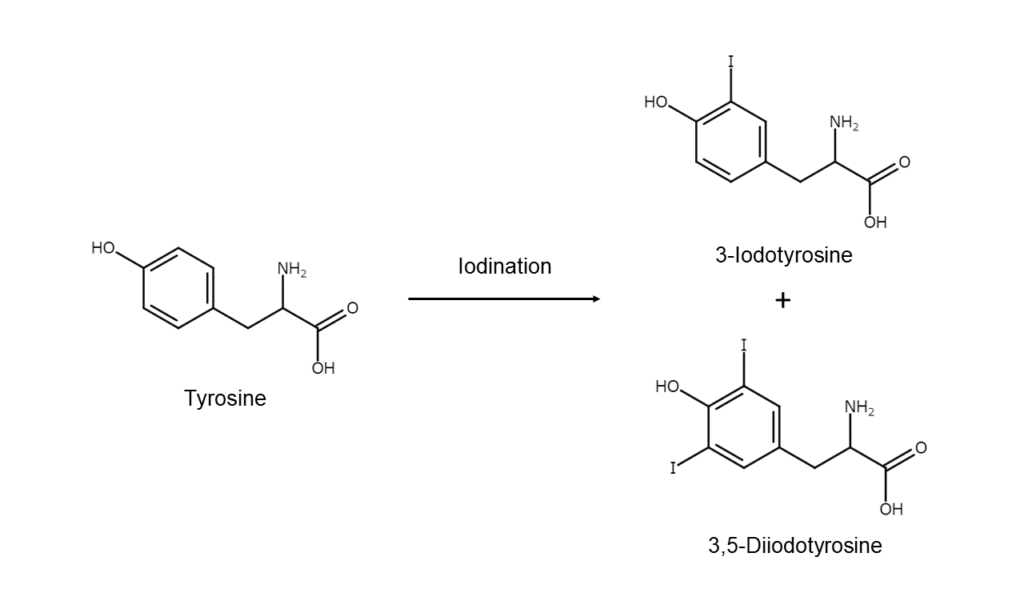
In this way, the cationization of iodine, the iodination of aromatics, and other aromatic compound iodination processes introduced so far have parallel mechanisms inside the body. Here we can see that the role fulfilled by oxidases within the body is covered by inorganic salts and oleum in the laboratory, as was discussed in this article.
Triiodothyronine and thyroxine generation through coupling
Tyrosine iodination generates monoiodotyrosine and diiodotyrosine, which then undergo oxidative coupling with peroxidase and hydrogen peroxide. This coupling creates triiodothyronine (T3) and thyroxine (T4).

The generated triiodothyronine (T3) and thyroxine (T4) are then stored in the follicular lumen for a certain time before being recollected by the follicular epithelial cells and secreted into the blood. Normally, it is mostly T4 that is made in the thyroid. The thyroid also produces T3, but this amounts to a tenth of the T4 it produces. Other organs, such as the liver, produce the remaining T4 in the body. The T3 produced in organs is not newly made from square one. The T4 carried to organs is stripped of one iodine atom through the action of deiodinase enzymes, a process that produces T3. A portion of the removed iodine is reused while the remainder is excreted. In industrial settings, iodine is similarly collected and reused because of its high cost, just as iodine is partially reused in the body.
This illustrates the commonalities between synthesis mechanisms for substances in the body and the mechanisms used for chemical synthesis. Iodine is not only helpful in making halides with its ease of use but is also crucial in its role in maintaining the functions of our bodies.
This article concludes our introduction of aromatic compound iodination with elemental iodine.
Starting with the next article, we will explore the iodination of aliphatic compounds using elemental iodine.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing. (Japanese)
2) Barker, I. R. L., Waters, W. A. J. Chem. Soc., 1952, 150.
3) Haszeldine, R, N., Sharpe, A. G. J. Chem. Soc., 1952, 993.
4) Stoll, A., H., Knochel, P. Org. Lett., 2008, 10, 113.
5) Koradin, C., Dohle, W. et al. Tetrahedron, 2003, 59, 1571.
6) Fletcher, T. L., Namkung, M. J. et al. J. Org. Chem., 1960, 25, 1342.
7) Rahman, Md. A. Shito, F. et al. Synthesis, 2010, 27.
8) Rozen, S., Zamir, D. J. Org. Chem., 1990, 55, 3552.








