
Alkane iodination: Aliphatic compound iodination overview and reactions: Aliphatic compound iodination reactions (1): Discussion series on bromination/iodination reactions 18
Several of our previous articles in this series have covered aromatic compound iodination reactions with elemental iodine. Starting with this article, we will discuss similar aliphatic compound iodination reactions with elemental iodine.
The single term “aliphatic compound” encompasses a great variety of types, including alkanes, alkenes, and alkynes. Unsurprisingly, the iodination reaction characteristics of each of these compounds also vary significantly. To master aliphatic compound iodination, it is crucial to organize the similarities and differences between each reaction and understand them systematically.
In this article, we first give a digest of various aliphatic compound iodination reactions before providing details on the first reaction on the list: alkane iodination.
■ What you can learn from this article ✔ Elemental iodine has low reactivity, and the resulting iodoalkanes are unstable against heat, light, and acid catalysts, making the direct iodination of alkanes challenging. ✔ Direct iodination with elemental iodine is possible in the presence of an oxidant or under conditions that promote chain radical generation. However, yields are low, and harsh reaction conditions are required, limiting the range of applicable compounds. ✔ In the pharmaceutical industry, diiodomethane is utilized to form cyclopropane skeletons within active pharmaceutical ingredients (API). ■ Recommended Articles ・ Iodine addition to alkenes: Aliphatic compound iodination reactions (2): Discussion series on bromination/iodination reactions 19 ・ Iodination of carboxylic acid, nitro compounds, and other inactive aromatic compounds: Aromatic compound iodination reactions (3): Discussion series on bromination/iodination reactions 17
contents
Describing the aliphatic compound iodination with elemental iodine
Moving forward with this series, we will now discuss aliphatic compound iodination in the order below.
(“① Alkane iodination” is covered in the latter part of this article, while the remaining reactions (“② Iodine addition to alkenes” through “⑤ Iodination of carboxylic acid and related compounds”) will be discussed in the next and other upcoming articles.)
①Alkane iodination
Generally, directly iodinating alkanes using elemental iodine is not an easy feat. However, in some cases, adding an oxidant or generating radicals can enable direct iodination.
②Iodine addition to alkenes
The addition of iodine to alkenes occurs through ionic or radical mechanisms. However, because these reactions are slow and the resulting products are unstable, this approach has limited use as a synthesis method.
③Alkyne iodination
There are two typical approaches for alkyne iodination. The first approach adds two iodine atoms to an acetylene bond, while the second approach brings about iodination of the terminal alkyne carbon. Both of these approaches feature relatively smooth reactions.
④Carbonyl compound iodination
Carbonyl compound iodination methods have drawbacks that include low yields, susceptibility to steric hindrances, and poor suitability for mass synthesis. However, several improved methods have been explored and reported with smoother reactions in recent years.
⑤Iodination of carboxylic acid and related compounds
Once reaction conditions are arranged, heating carboxylic acid together with elemental iodine allows α-iodocarboxylic acid and α-iodoacyl chloride to be obtained. Reactions that result in α-iodoacyl chloride are predicted to occur in two stages, the first being the generation of a ketene and the second being the addition of iodine to the ketene.
Aliphatic compound iodination reactions with elemental iodine: Alkane iodination
Direct iodination with elemental iodine generally challenging
On top of elemental iodine having low reactivity, iodoalkane reaction products are not very stable against various exposures, including heat, light, and acid catalysts. For these reasons, unlike chlorides or bromides, alkanes are generally challenging to iodinate directly with elemental iodine.
Therefore, when synthesizing iodoalkanes, indirect methods that use halogenated alkanes as starting materials are generally used. The following methods are examples.
- Methods that substitute a functional group, such as a chlorine, bromine, or ester group, with an iodine ion.
- Methods where elemental iodine is set to act on an organometallic compound or an enolate anion.
※To learn more about these methods, check the article below
Achieving direct iodination by leveraging oxidants and radicals
Does that mean that the direct iodination of alkanes with elemental iodine is nothing more than an impossible dream?
On the contrary, direct iodination using elemental iodine is possible under the presence of an oxidant or under conditions that result in a chain of radical generation. However, in general, yields are low and harsh reaction conditions are required, limiting the compounds that can be used.
In order to overcome these issues in the laboratory, photoexcitation or electron transfers are utilized to generate radicals under mild reaction conditions. Taking this approach and capturing the generated radicals with iodine enables the iodination of alkyl chains.
Certain cycloalkanes readily react with elemental iodine
Compounds known as cycloalkanes are like alkanes in that they do not readily react with elemental iodine. However, cyclopropane has been reported to easily undergo iodination cleavage, even at room temperature, changing into a 1,3-diiodine compound2.
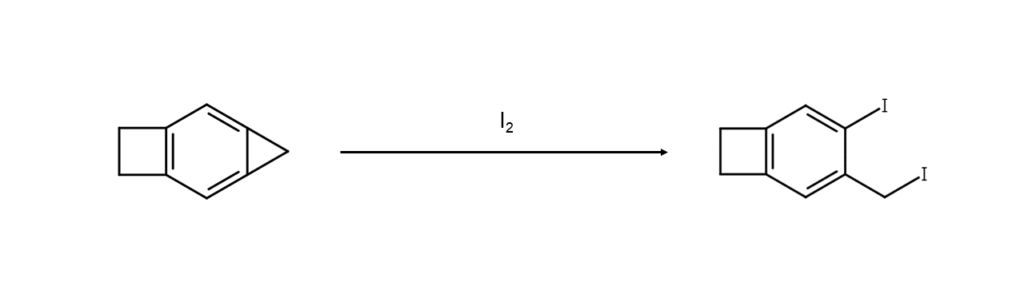
There is also research demonstrating that combining elemental iodine with sodium tert-butoxide allows for the iodination of cyclohexane under heated reaction conditions without external light stimulation3. The research reports improved reaction reproducibility and yield with the addition of 20 mol% bromine.

Column 1: Iodomethane as a pesticide
Iodoalkanes are generated by alkane iodination, the main topic of this article. Take methane, the simplest of the alkanes, and replace one of its hydrogen atoms with an iodine atom to obtain a compound known as iodomethane.
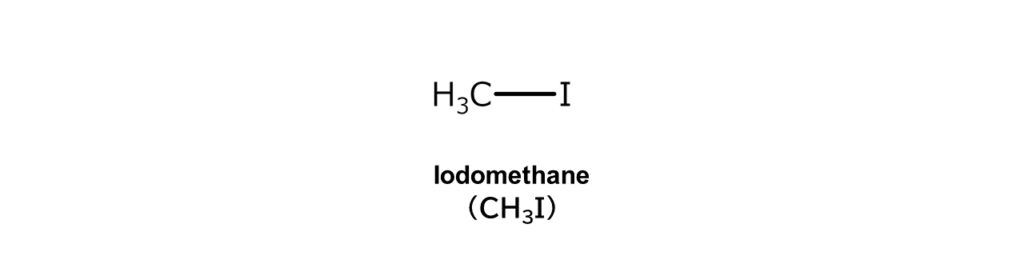
Did you know that this iodomethane is actually used as a pesticide?
The action of iodomethane to kill targeted pests (such as harmful insects and pathogens) is postulated as follows. First, iodomethane permeates the soil or the inner parts of a crop. The structural components of the targeted pests in the soil or crop then nucleophilically attack the iodomethane (nucleophilic substitution reaction). This causes the essential enzymes inside targeted pests to cease functioning, resulting in the death of the targeted organisms.
We see here that iodomethane supports our everyday meals from behind the scenes.
Column 2: A surprising connection between diiodomethane and pharmaceuticals
Going one step further, take iodomethane and replace yet another hydrogen atom with iodine to get diiodomethane.
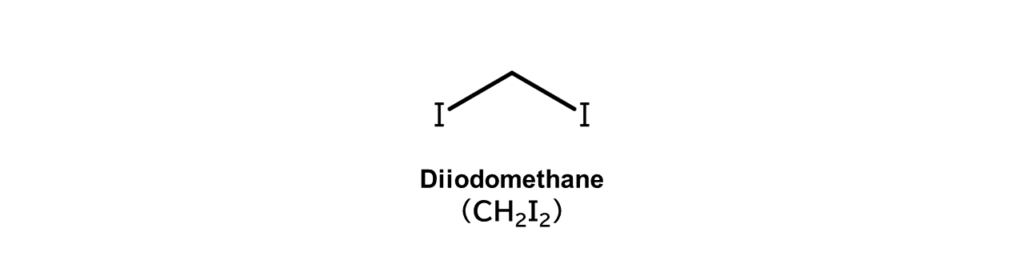
Diiodomethane has many different applications. For example, diiodomethane is used as a reagent in the cyclopropanation of alkenes, as shown in the following reaction (Simmons-Smith reaction).

Cyclopropane skeletons, such as the example shown above, can be found in many active pharmaceutical ingredients (API). These cyclopropane skeletons play a crucial role in effectively bonding APIs with target proteins. For this reason, API manufacturers utilize diiodomethane to form cyclopropane skeletons in API compounds.
At MANAC, our business includes comprehensive, one-stop commissioned services for API manufacturers, from the provision of diiodomethane to the performance of cyclopropanation reactions, as well as the collection and recycling of resulting iodine byproducts. Find out more in the article below.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing. (Japanese)
2) Davalian, D., Garratt, P. J. J. Am. Chem. Soc., 1975, 97, 6883.
3) Montoro, R., Wirth, T. Synthesis, 2005, 1473.












