Iodoalkane synthesis from alcohols: Iodination reactions using alkali iodides (2): Discussion series on bromination/iodination reactions 25
In the previous issue of this series, we began our discussion on iodination reactions that use alkali iodides, commonly found as sodium iodide and potassium iodide. Since alkali iodides are widely utilized as iodinating reagents, knowledge of their characteristics and applications is greatly important.
In this issue, we cover methods to synthesize iodoalkanes from alcohols using alkali iodides. While these methods are somewhat complex due to reaction conditions that vary depending on the structure and properties of the alcohol, we will break them down for further understanding in our discussion. Be sure to read to the end.
contents
Iodination reactions using alkali iodides: Iodoalkane synthesis from alcohols
①Reaction methods under the presence of acid
Methods with strong protonic acids
Reacting an alcohol and an alkali iodide under the presence of a strong protonic acid generates high yields of iodoalkanes. Such methods are widely used owing to a low occurrence of side reactions.
The reaction mechanism differs depending on the structure of the reactant alcohol. When a primary or secondary alcohol is used, protonation with an acid generates oxonium ion intermediates ([ROH2]+), which undergo an SN2 attack by iodine ions, resulting in a product with inversed stereochemistry. Conversely, caution should be taken when using tertiary alcohols as alkene generation through elimination reactions and the isomerization of carbon skeletons readily occur due to an SN1 mechanism in the reaction via stable carbocations.
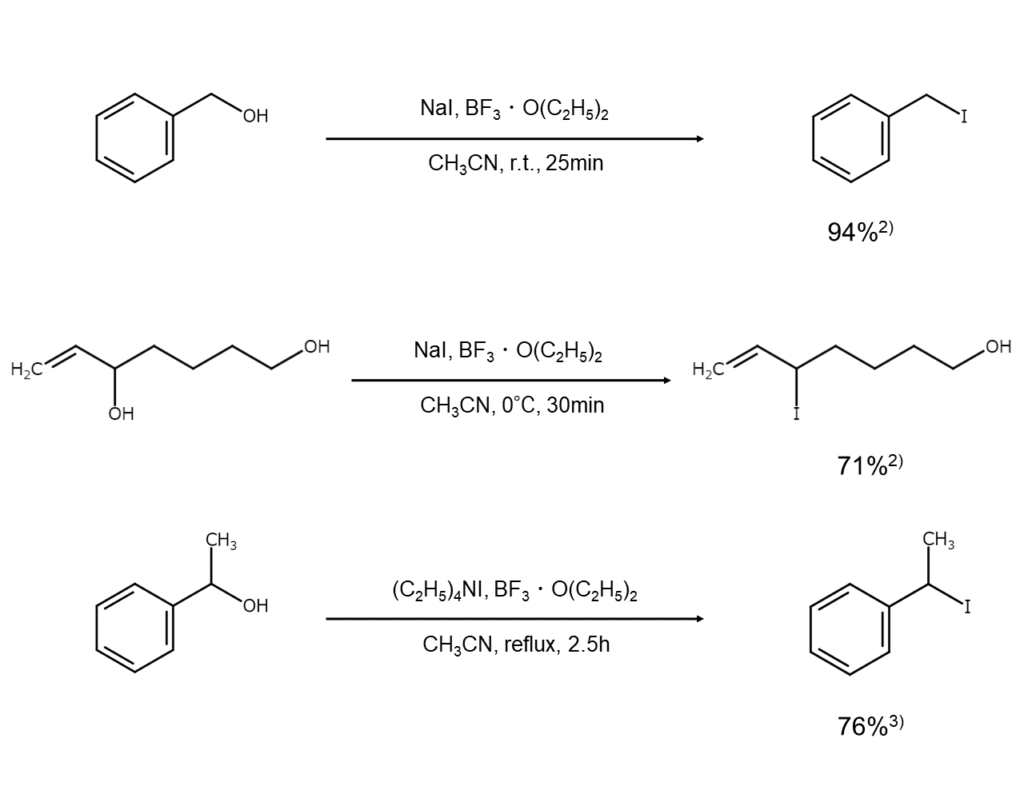
Methods that use BF3·O(C2H5)2
Some proposed methods use BF3·O(C2H5)2 as a reagent2), 3). In contrast to tertiary, ally, and benzyl alcohols that rapidly convert into iodides, primary and secondary alcohols will slow the reaction, making them useful when targeting the partial iodination of a polyhydric alcohol.
Other improved methods
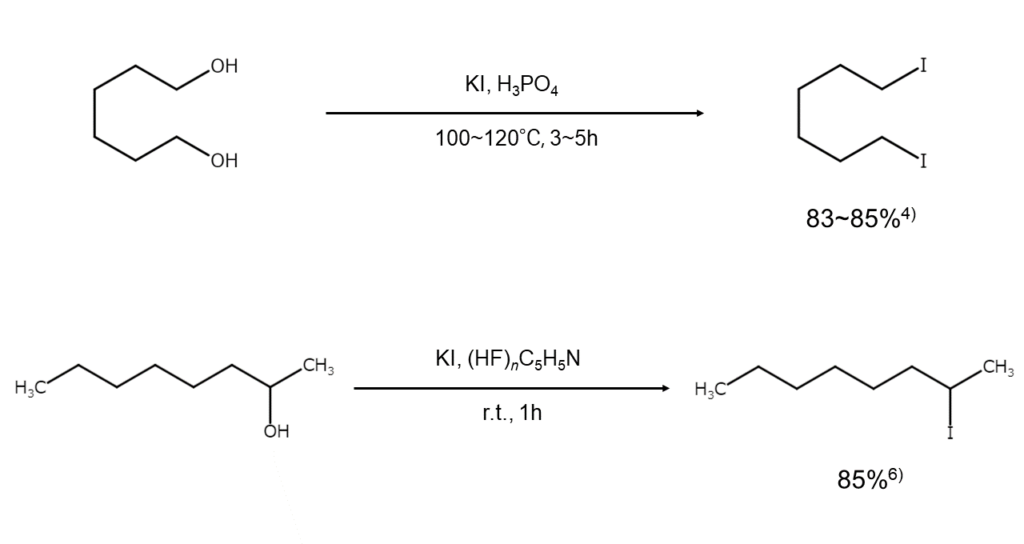
There are promising proposals for improved methods that effectively apply an extensive range of aliphatic and alicyclic alcohols to achieve high yields without isomerization or racemization. For example, some methods use certain combinations with the alcohol, such as with KI-phosphate4), NaI-chlorotrimethylsilane5), and KI-pyridine/polyhydrofluoric acid6).
Further, because the polyfluoroalcohol RfCH2OH does not easily react with HI or hydroiodic acid, methods that first convert it into phosphate ester with POCl3 and then react it with NaI at room temperature are used7). (See the formulas below.)
3 RfCH2OH + POCl3 → (RfCH2O)3P=O + 3 HCl
(RfCH2O)3P=O + 3 NaI →3 RfCH2I + Na3PO4
②Methods via esters (reaction methods under neutral conditions)
In the case of acid-unstable alcohols, a basic approach entails an initial conversion, such as into a toluenesulfonic acid ester, methanesulfonic acid ester, or trimethylsilyl ether, followed by a reaction with an alkali iodide under neutral conditions. Typical methods heat a combination with NaI or KI under reflux in a solution of acetone, 2-butanone, DMF, or similar (see the reaction formulas below). The resulting products have inverted configurations, which characterize these methods as they occur through an SN2 mechanism.
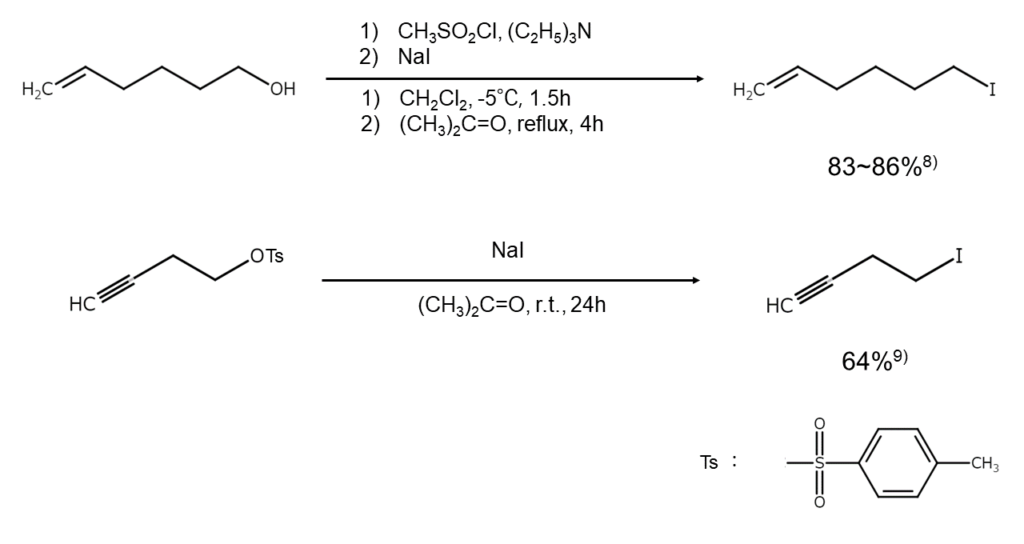
In the case of esters of cyclic alcohols, which require milder reaction conditions, it is recommended to use methods that carry out a reaction with MgI2 or CaI2 in ether10). While these methods can also be used with enol esters11), they do not work well with esters of tertiary alcohols.
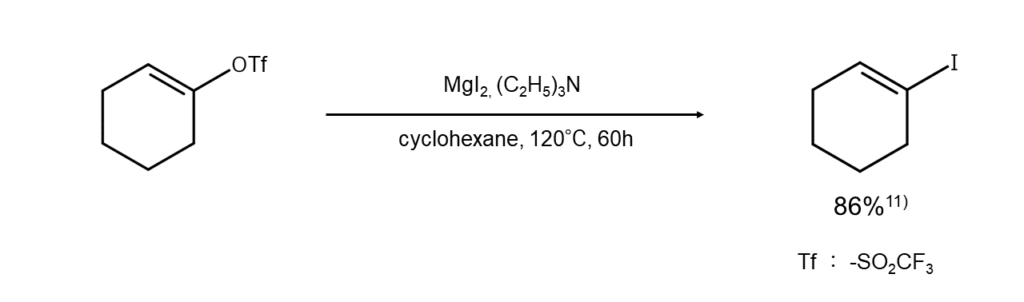
Column 1: Addressing iodine deficiency with supplements that contain alkali iodide
You have likely heard of thyroid hormones, but did you know that these substances are crucial for regulating a multitude of biochemical reactions, such as those for protein synthesis and enzymatic activity? Iodine is essential for the synthesis of thyroid hormones, so an iodine deficiency in the body will cause various issues.
A simple way of addressing iodine deficiency is by taking supplements. In fact, many supplements contain iodine in the form of potassium iodide or sodium iodide12). Research has demonstrated that potassium iodide is almost completely absorbed in humans13).
As we can see, alkali iodides are an effective means to protect our bodies from iodine deficiency.
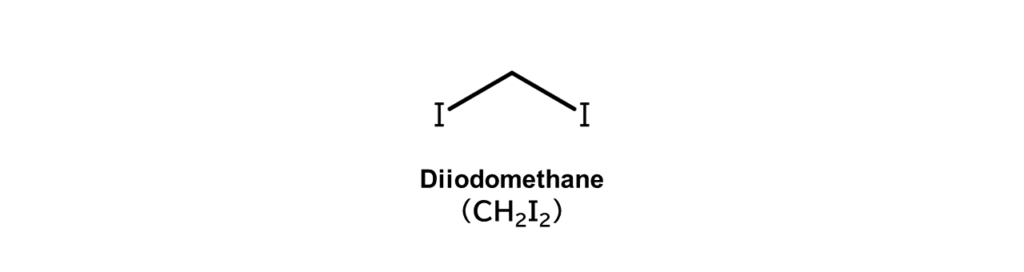
Column 2: Diiodomethane in the pharmaceuticals field
The reactions covered in this article produce iodoalkanes, an example being diiodomethane, which we touch upon here in Column 2.
Diiodomethane has many different applications. For example, diiodomethane is used as a reaction reagent in alkene cyclopropanation reactions, as shown below (Simmons-Smith reaction).
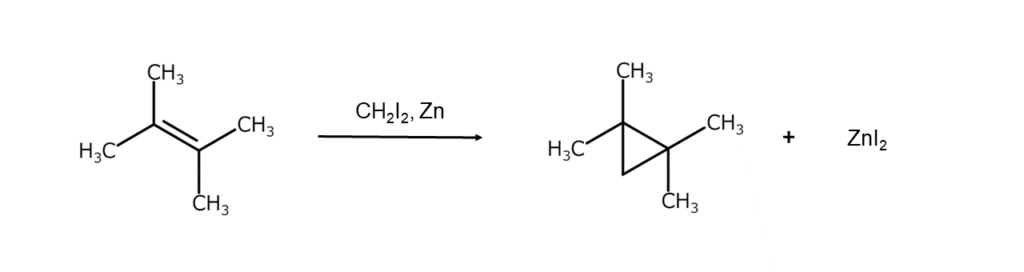
Cyclopropane skeletons, such as in the example above, can be found in many active pharmaceutical ingredients (API). These cyclopropane skeletons play a crucial role in effectively bonding APIs with target proteins. For this reason, API manufacturers utilize diiodomethane to form cyclopropane skeletons in API compounds.
At MANAC, our business includes comprehensive, one-stop commissioned services for API manufacturers, from the provision of diiodomethane to the performance of cyclopropanation reactions, as well as the collection and recycling of resulting iodine byproducts. Find out more in the article below.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing. (Japanese)
2) Vankar, Y. D., Rao, C. T. Tetrahedron Lett., 1985, 26, 2717.
3) Mandal, A. K., Mahajan, S. W. Tetrahedron Lett., 1985, 26, 3863.
4) Stone, H., Schechter, H. Org. Synth. Coll. Vol. IV, 323 (1963).
5) Olah, G. A., Narang, S. C. et al. J. Org. Chem., 1979, 44, 1247.
6) Olah, G. A., Welch, J. Synthesis, 1974, 653.
7) Krogh, L. C., Reid, T. S. et al. J. Org. Chem., 1954, 19, 1124.
8) Bailey, W. F., Luderer, M. R. et al. Org. Synth. 81, 121 (2005).
9) Owen, L. N., Saharia G. S. J. Chem. Soc., 1953, 2582.
10) Place, P., Roumestant, M. L. et al. Bull. Soc. Chim. Fr., 1976, 169.
11) Martinez, A. G., Alvarez, R. M. et al. Synthesis, 1986, 222.
12) National Institutes of Health. Dietary Supplement Label Database 2020.
13) Aquaron, R., Delange, F. et al. Cell. Mol. Biol., 2002, 48, 563.