
Active/inactive aromatic ring bromination: Bromination reactions that use NBS (2): N-bromo compounds (4): Discussion series on bromination/iodination reactions 4
In this series, we discuss bromination and iodination reactions, specialties of MANAC. This article discusses various NBS bromination reactions in continuation to the last issue.
We will cover aromatic ring bromination in this issue. Depending on reaction conditions, the use of NBS allows for the bromination of both aromatic rings activated by electron-donating groups and aromatic rings deactivated by electron-withdrawing groups. Basic principles behind reactions, solvent effects, and examples of reactions are discussed in this article as a helpful reference to our readers.
■ What you can learn from this article ✔ The bromine atom in NBS is positively polarized, acting electrophilically on electron-rich compounds. ✔ The regioselectivity of bromination reactions depends significantly on the polarity of the solvent. ✔ To overcome reaction challenges that conventional techniques cannot resolve, MANAC is advancing research on microflow synthesis. ■ Recommended Articles ・ Addition of bromine to alkenes, bromohydrination of alkenes, synthesis of bromoalkanes from alcohols: NBS bromination reactions (3): N-bromo compounds (5): Discussion series on bromination/iodination reactions 5 ・ Allylic position and benzylic position bromination: Bromination reactions that use NBS (1): N-bromo compounds (3): Discussion series on bromination/iodination reactions 3
contents
Describing N-bromosuccinimide (NBS)
A very commonly used, easy-to-handle brominating agent
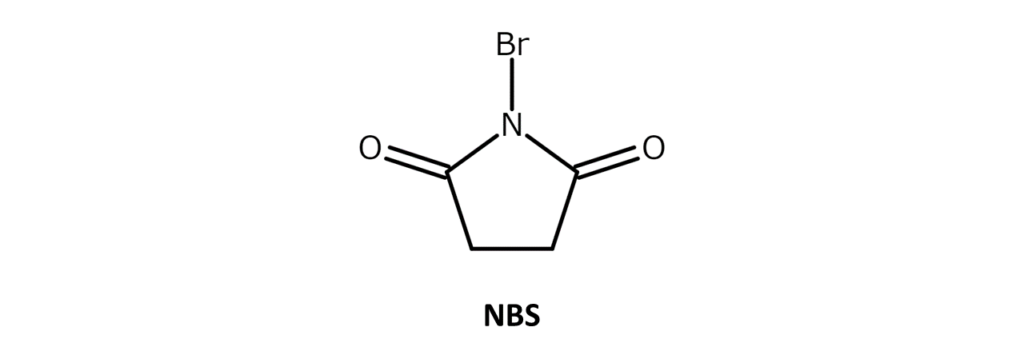
NBS is a white to slightly yellow crystalline powder with a weak bromine odor and a melting point of 173–176°C (decomposition). The compound is soluble in substances such as acetone, THF, DMF, acetonitrile, and DMSO, slightly soluble in water and acetic acid, and has poor solubility in hexane and carbon tetrachloride.
NBS powder is easier to handle than bromine (liquid), making it a common first-line brominating agent in organic syntheses. With its ability to undergo long-term storage in cool, dark, dry places, NBS is also relatively inexpensive.
This article covers information regarding the characteristics and precautions of NBS and an overview of bromination reactions that use NBS.
Bromination reactions that use NBS: active aromatic ring bromination
Reaction details
The nitrogen atom in NBS sits adjacent to an electron-withdrawing carbonyl group. This means that the bromine atom in NBS is positively polarized and acts electrophilically on compounds rich in electrons.
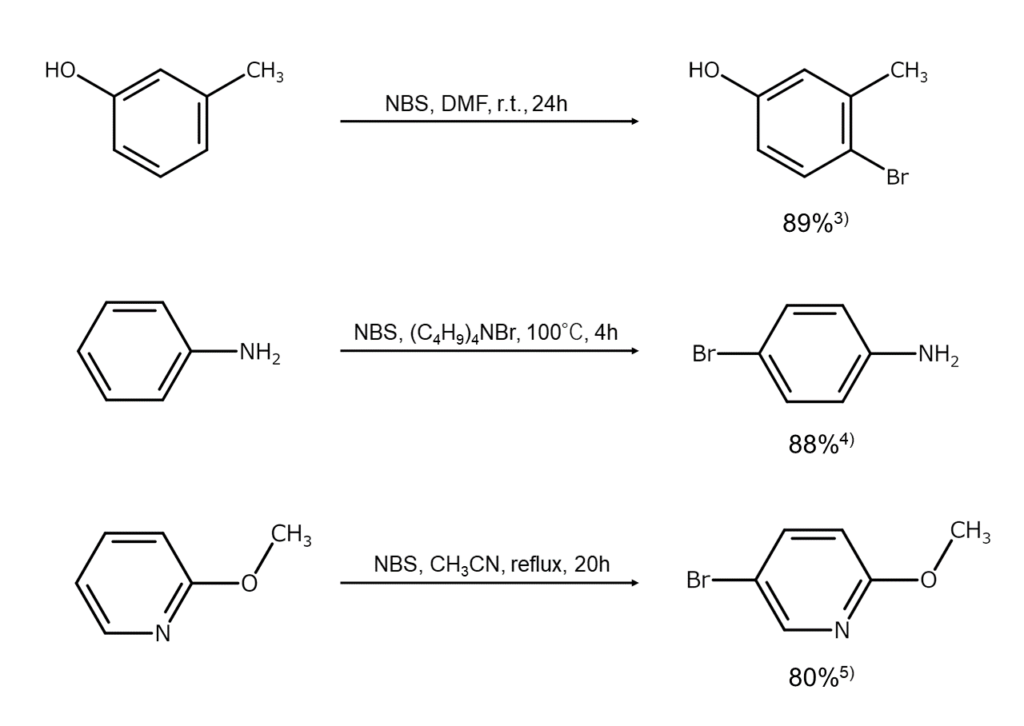
In this article’s active aromatic ring bromination reactions, NBS brominates aromatic rings moderately activated by electron-donating groups and electron-rich heteroaromatic rings by acting electrophilically on them (aromatic electrophilic substitution reaction).
The level of aromatic ring activation by the electron-donating group must be moderate for this reaction to progress smoothly. When aromatic rings become excessively activated by strong activating groups such as hydroxy and amino groups, they become susceptible to oxidation or resinification due to the action of NBS. However, even in such cases, bromination can generally be successfully carried out if the hydroxy or amino group is switched to an ester or amide group for protection.
The solvent largely determines the regioselectivity of the bromination reaction. The table below shows bromination results2 under various reaction solvents using NBS for 3-substituted aniline, which contains inactive groups such as chloro and nitro groups. These results illustrate how the regioselectivity of these reactions is greatly dependent on the solvent polarity.
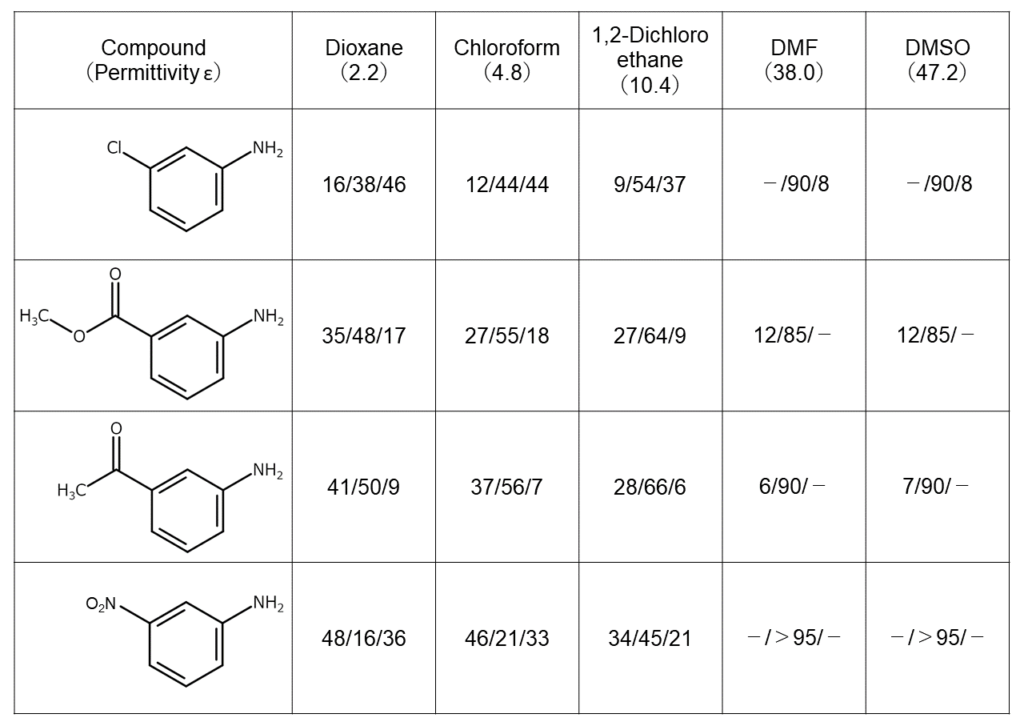
Numbers indicate bromide isomeric ratios at the 2nd, 4th, and 6th positions, in order from left to right.
Examples of NBS bromination reactions for active aromatic rings are shown below.
[MANAC Tidbits] Controlling bromination reaction regioselectivity through microflow synthesis
Conditions required for reaction progress can be found relatively easy in most cases to ensure activated aromatic rings brominate even under moderate conditions. This is not the case, however, for active aromatic rings with multiple reaction points (positions where bromination can occur). To selectively brominate specific positions on such aromatic rings, precise reaction conditions must be set, including the type and amount of brominating agent or solvent to use, the reaction temperature, and the method of addition. There are certainly cases in which these considerations fail to yield the targeted reaction selectivity.
In seeking ways to overcome the limitations of traditional technologies for challenges in bromination and other reactions, MANAC continues to advance its research on microflow synthesis.
Bromination reactions that use NBS: inactive aromatic ring bromination
Reaction details
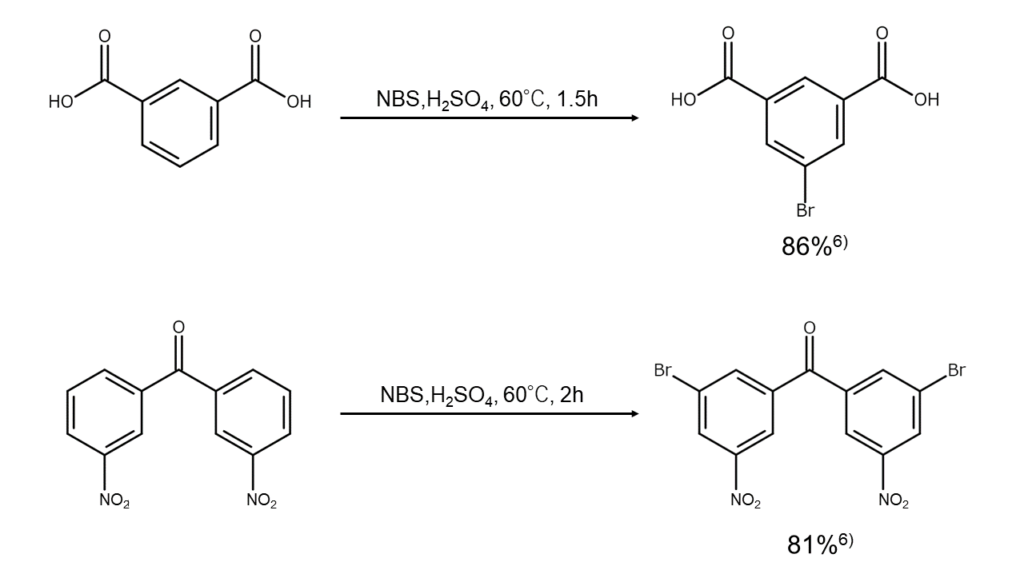
Aromatic rings deactivated by electron-withdrawing groups do not readily undergo electrophilic substitution reactions with NBS, so bromination largely fails to progress under moderate conditions. However, the use of sulfuric acid as a solvent allows for bromination.
The examples below illustrate NBS bromination reactions for inactive aromatic rings.
[MANAC Tidbits] How should sulfuric acid solvent be disposed of?
Sulfuric acid is highly useful in synthesis involving the bromination of inactive aromatic rings that contain a nitro group, a strong deactivating group, since it can introduce bromine into the meta position. But from an industrial point of view, this reaction may not be the best. This stems from the common problem following each reaction of how to dispose of the large volume of sulfuric acid used as a reaction solvent. The disposal methods for solvents, among other aspects, must be included within the comprehensive consideration of each industrial reaction design.
MANAC manufactures and sells NBS and DBDMH, two common N-bromo compounds. Please feel free to inquire.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing
2) Bartoli, S., Cipollone, A. et al. Synthesis, 2009, 1305.
3) Mitchell, R. H., Lai, Y.-H. et al. J. Org. Chem., 1979, 44, 4733.
4) Ganguly, N. C., De, P. et al. Synthesis, 2005, 1103.
5) Canibano, V., Rodriguez, J. F. et al. Synthesis, 2001, 2175.
6) Rajesh, K., Somasundaram, M. et al. J. Org. Chem., 2007, 72, 5867.
















