
A first-line brominating agent:describing N-bromosuccinimide (NBS): N-bromo compounds (2): Discussion series on bromination/iodination reactions 2
In this series, we discuss bromination and iodination reactions, both specialties of MANAC. This issue covers N-bromosuccinimide (NBS), the N-bromo compound most commonly used as a brominating agent.
NBS is often used as a first-line brominating agent in laboratories and other such facilities. There are likely many who have experience using NBS, but are unfamiliar with its exact properties or handling methods.
In this article, we review details such as the characteristics of NBS, handling precautions, and an overview of bromination reactions that use NBS. Information in this article is provided as a reference to aid in a more efficient and safer utilization of NBS.
■ What you can learn from this article ✔ NBS is frequently used in organic synthesis as a brominating agent and is valued for its ease of handling. ✔ Although generally stable, NBS can decompose explosively if exposed to heat, impact, or friction, so protective gear and careful handling are essential to avoid contact with eyes and skin. ✔ NBS effectively brominates allylic and aromatic positions, and the byproduct succinimide is easy to separate from the reaction mixture due to its low solubility in carbon tetrachloride. ■ Recommended Articles ・ Allylic position and benzylic position bromination: Bromination reactions that use NBS (1): N-bromo compounds (3): Discussion series on bromination/iodination reactions 3 ・ Highly selective yet gentle brominating agents: N-bromo compounds (1): Discussion series on bromination/iodination reactions 1
contents
Describing N-bromosuccinimide (NBS)
A very commonly used, easy-to-handle brominating agent
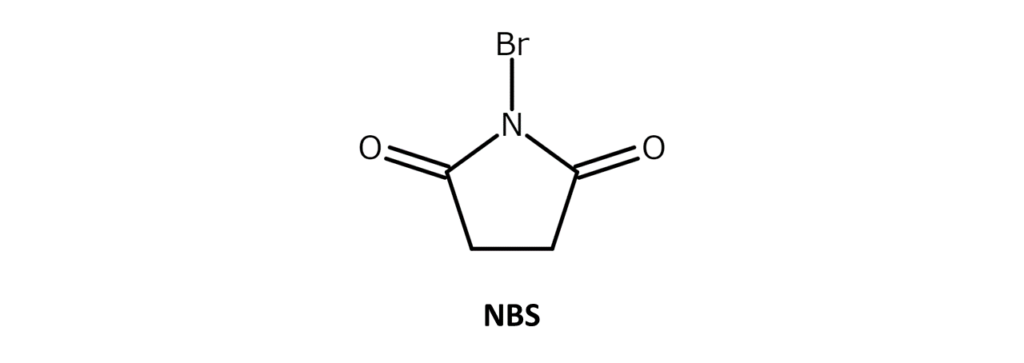
NBS is a white to slightly yellow crystalline powder with a weak bromine odor and a melting point of 173–176°C (decomposition). The compound is soluble in substances such as acetone, THF, DMF, acetonitrile, and DMSO, slightly soluble in water and acetic acid, and has poor solubility in hexane and carbon tetrachloride.
NBS powder is easier to handle than bromine (liquid), making it a common first-line brominating agent in organic syntheses. With its ability to undergo long-term storage in cool, dark, dry places, the price of NBS is also relatively stable.
Industrial-grade chemicals produced in Japan with a 98%–99% purity can be purchased from MANAC.
NBS handling precautions
NBS can safely be handled under normal conditions. However, NBS may violently decompose if subjected to rapid heating, impacts, or friction. When handling, care should also be taken to prevent NBS from coming into contact with eyes, skin, or mucous membranes. Contact with eyes may lead to blindness, and skin contact may result in skin irritation.
When handling NBS, wear protective equipment such as particulate respirators, safety glasses, and rubber gloves.
Bromination reactions with NBS
What kind of bromination reactions use NBS?
NBS is used in the bromination reactions below, and has long been particularly famous as a reagent for the Wohl-Ziegler reaction.
———————————–
・Allylic position and benzylic position brominations (Wohl-Ziegler reaction)
・Active aromatic ring bromination
・Inactive aromatic ring bromination
・Addition of bromine to alkenes, bromohydrination of alkenes
・Synthesis of bromoalkanes from alcohols
・Bromination of the alpha position in a carbonyl group
・Bromination of carboxylic acids and related compounds
・Synthesis of bromoallene from propargyl alcohol
・Aromatic ring ortho-selective bromination
———————————–
Bromination reactions result in a large amount of succinimide. With its poor solubility in carbon tetrachloride, succinimide floats at the top of the liquid, making it easy to separate out. Besides carbon tetrachloride, cyclohexane and benzene can also be used as reaction solvents.
The bromination position changes depending on the reaction conditions. For example, using polar solvents in place of carbon tetrachloride, such as nitromethane or tetrachloroethane, helps facilitate the addition of bromine to carbon-carbon double bonds. In recent years, with strong concerns over carbon tetrachloride’s health and environmental risks, methyl acetate2 and benzotrifluoride3 have also been proposed as alternative reaction solvents.
In addition to its use in bromination reactions, NBS has a wide range of applications, including the dehydrogenation and oxidation of hydroaromatic compounds and peptide bond cleavage. NBS has also reportedly been researched as an antifogging agent for photographs in the past.
Precautions for using NBS in bromination reactions
When bromination reactions progress slowly, the addition of benzoyl peroxide (BPO) or azobisisobutyronitrile (AIBN) and the application of photo-irradiation is effective. However, this may lead to rapid heating and trigger a violent reaction. Caution is required when conducting large-scale synthesis reactions.
[MANAC Tidbits] NBS is not actually pale yellow?
If you are a chemist, you have used NBS at least once before. Most would likely describe its appearance as a pale yellow, but this is actually from the bromine given off by NBS. As time passes after purchasing NBS, its decomposition results in the release of bromine.
Yellowed NBS can be purified by dissolving it in heated acetic acid or hot water, cooling it, collecting the formed crystals, and drying the resulting product. NBS is best stored in a cool, dark place. (Do not store NBS in metal containers.)
The amount of released bromine contained in old NBS is minuscule and is highly unlikely to significantly impact selectivity in bromination reactions. However, in cases where precise bromination reactions are needed, it may be ideal to carefully time the purchase of NBS.
MANAC manufactures and sells NBS and DBDMH, two common N-bromo compounds. Please feel free to inquire.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing
2) Amijs, C. H. M., van Klink, G. P. M. et al. Green Chem., 2003, 5, 470.
3) Suarez, D., Laval, G. et al. Synthesis, 2009, 1807.
4)Fuji Photo Film Inc., Ger. Offen. 2, 326, 865(1973)













