
Iodyl compounds as oxidants and other applications: Hypervalent organoiodine compounds (5): Discussion series on bromination/iodination reactions 33
Throughout this series, we have reviewed many different hypervalent organoiodine compounds, including dihalogenoiodo compounds, (diacyloxyiodo)arenes, iodonium compounds, iodosyl compounds, and iminoiodo compounds.
In this article, we finish our review of these hypervalent organoiodine compounds with a finale featuring iodyl compounds.
Iodyl compounds are useful and have various applications, including as oxidants.
However, they must also be handled with care as they are high-energy compounds that may explode under certain conditions.
As this overview of iodyl compounds also covers handling methods in addition to compound characteristics and synthesis methods, we highly recommend reading the entire article.
contents
Hypervalent organoiodine compounds
Organic compounds that contain hypervalent iodine
A hypervalent organoiodine compound is just that — an organoiodine compound that is hypervalent.
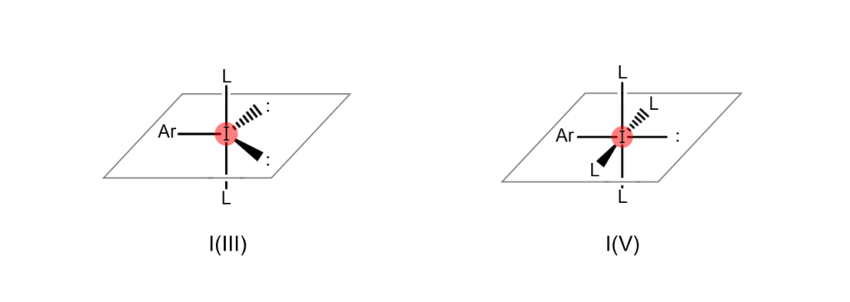
Atoms are “hypervalent” when the most outer electron shell (the part of the atom related to bonds with other atoms), which sits furthest from the nucleus, formally contains more than eight electrons. This state enables the atom to form a higher number of bonds with other atoms. For example, iodine atoms gain the ability to bond with multiple atoms at once when they become hypervalent, as shown in the figures below (the iodine atom on the left is trivalent, while the iodine atom on the right is pentavalent). When an organic compound contains hypervalent iodine, it is known as a “hypervalent organoiodine compound.”
Introducing hypervalent organoiodine compounds: Iodyl compounds
Iodyl compounds are defined by an iodyl functional group (-IO2). Careful handling is a must since these compounds are classified as high-energy compounds alongside azide and nitro compounds.
Here, we introduce three iodyl compounds that can be found in the laboratory.
Iodylbenzene – A common iodyl compound
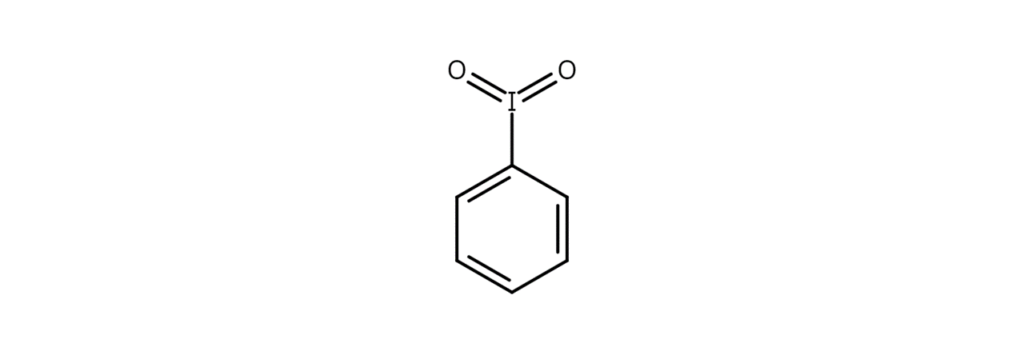
Iodylbenzene, alternatively called iodoxybenzene, is a crystalline solid with a melting point of 230°C. The compound explosively decomposes at temperatures of 250–253°C, warranting precautions such as ensuring that iodylbenzene is not heated without a solvent and only working with iodylbenzene inside a fume hood.
As it forms higher-order structures via association, the solubility of iodylbenzene in water is 2.8 g L-1 at 12°C and 12 g L-1 at 100°C. Iodylbenzene dissolves well in heated acetic acid but is insoluble or has poor solubility in common organic solvents. It is an amphoteric compound and will form salts with strong inorganic acids and alkali metals.
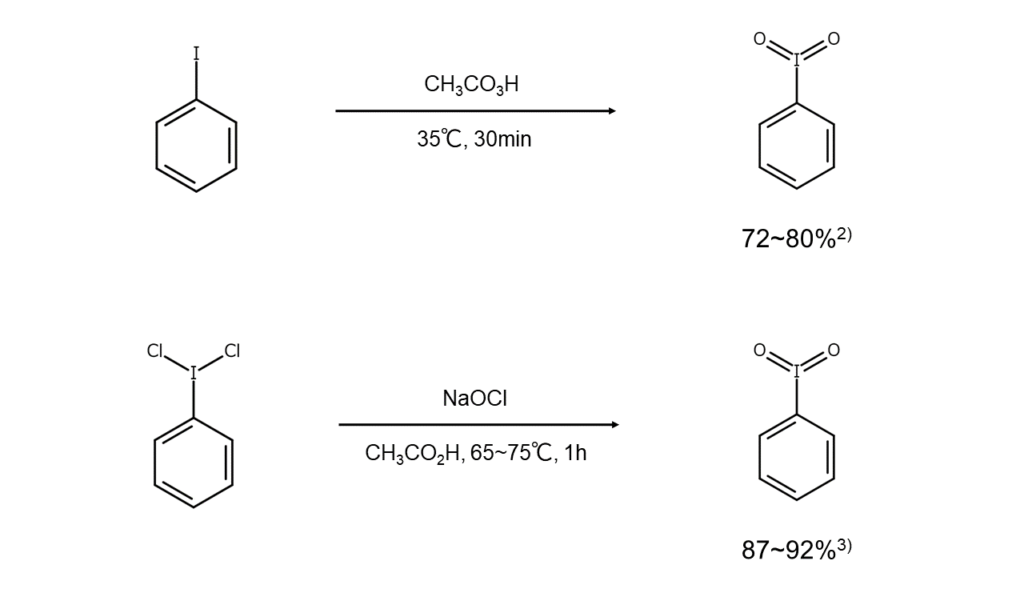
Because iodylbenzene is not commercially available, it is prepared in the lab as necessary by oxidizing an iodobenzene or an iodosylbenzene with a peracid or hypochlorous acid.
Here are a couple of iodylbenzene synthesis examples.
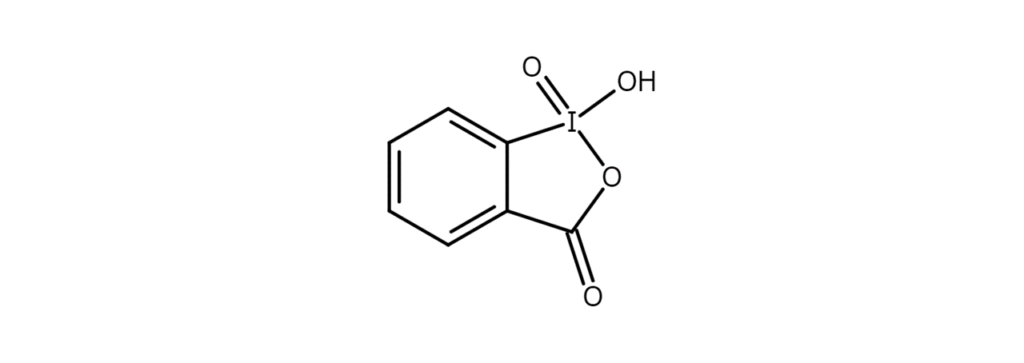
2-Iodylbenzoic acid (IBX) – A gentle oxidant and dehydrogenating agent
Second on the list of iodyl compound introductions is 2-iodylbenzoic acid (IBX), often used in the laboratory as a gentle oxidant or dehydrogenating agent.
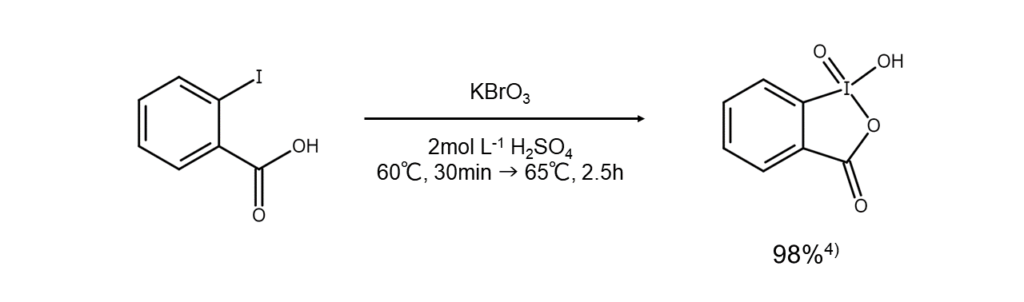
IBX is a compound that features a 1-hydroxy-1,2-benziodoxol-3(1H)-one-1-oxide heterocyclic structure (figure below). It is easily produced by oxidizing 2-iodobenzoic acid.
Owing to its relative stability and ease of use, IBX has a wide range of applications and is commercially available. However, caution is needed as IBX may explosively decompose if dried and subjected to impact.
The figure below is an example of IBX synthesis.
Dess-Martin reagent – Oxidizes alcohols into carbonyl compounds
The third iodyl compound introduction is Dess-Martin periodinane (DMP), a tri-O-acyl derivative of IBX (1,1,1-triacetoxy-1,1-dihydro-1,2-benzoiodoxol-3- (1H)-one) (figure below). DMP is used as a gentle oxidant to convert alcohols into carbonyl compounds.
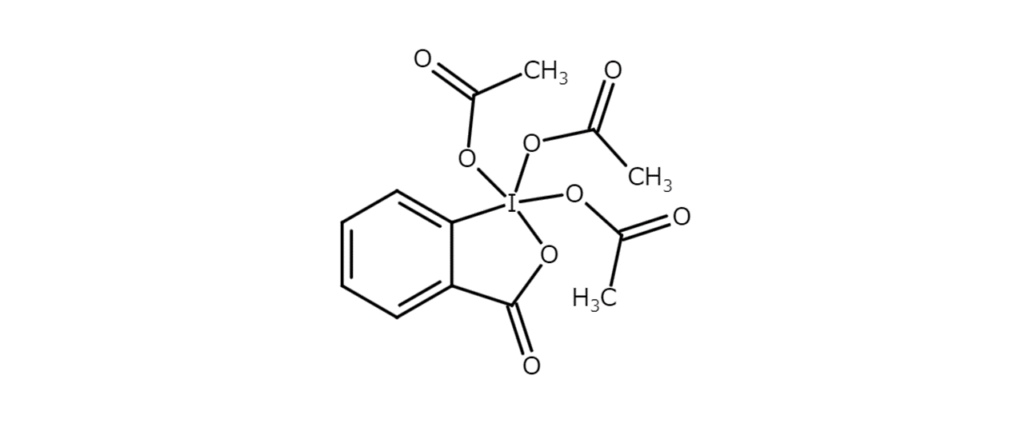
DMP is a white to slightly yellow crystalline powder with a melting point of 124°C (decomposition). The reagent is soluble in chloroform, acetone, acetonitrile, and dichloromethane, and is slightly soluble in ether and hexane.
While DMP is relatively stable and can be handled under normal conditions, there is a risk of fire or explosion if it comes into contact or is mixed with reducing agents or combustibles. Caution is also required regarding the generation of IBX (a compound that readily decomposes explosively when in a dry state) resulting from the hydrolysis of DMP. In fact, DMP was once available commercially, but has been pulled from the market due to accidents.
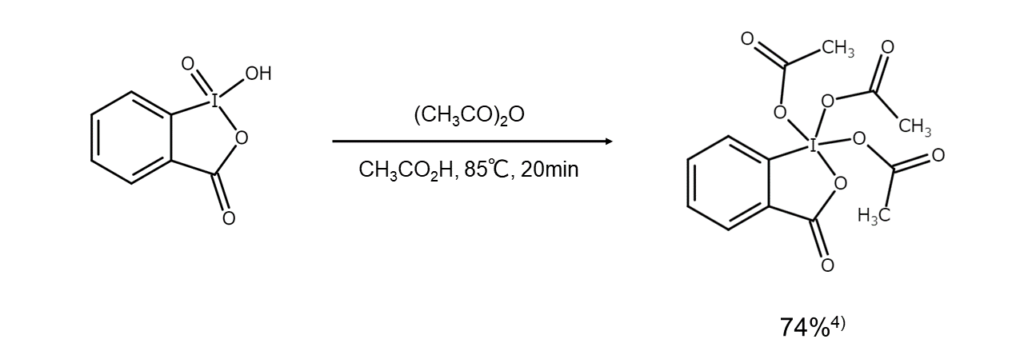
IBX is used to synthesize DMP. The following is an example of such synthesis.
Column: Making flexible OLEDs a reality with iodyl compounds
Organic light-emitting diode (OLED) technology is everywhere around us, from cellphones to televisions to device displays. While you may be familiar with these kinds of devices, did you know that the IBX and Dess-Martin reagent introduced in this article are related to the technology?
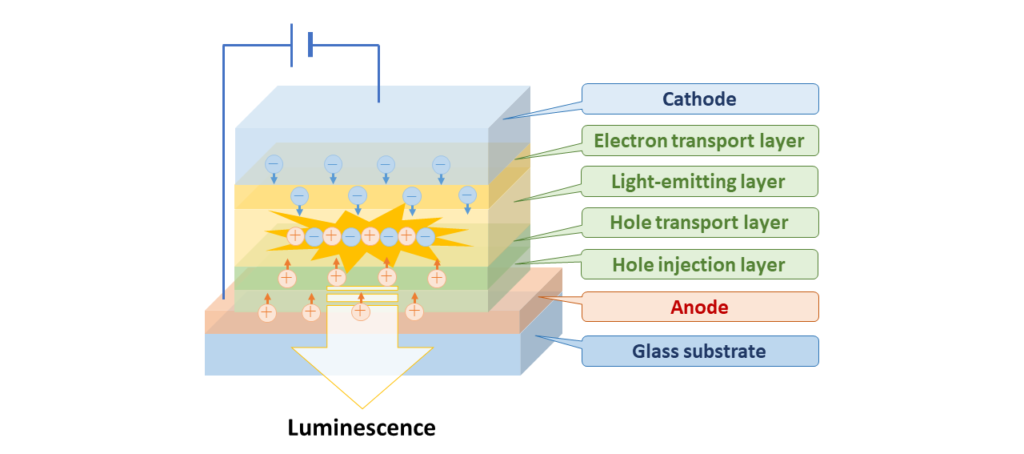
OLEDs utilize the organic material luminescence that occurs in response to electricity. They are comprised of electrodes (anode and cathode), a hole injection layer (a layer for injecting electron holes from the anode), electron and hole transport layers, an emissive layer, and other important components. This structure results in luminescence when the holes and electrons generated by the electrodes combine in the emissive layer.
Formation of the emissive layer, hole injection layer, and other such charge-transporting thin films requires drop-coating a substrate spinning at high speeds with a varnish (resin material dissolved in solvent) and then placing the coated substrate in high-temperature thermal annealing. However, in recent years, flexible and thin resin substrates are increasingly being used instead of traditional glass substrates. In turn, there is a growing need for technologies that can anneal the substrate at temperatures lower than conventionally needed in order to avoid damaging the resin substrate. The response to this need comes in the form of a patented technology (WO2018012416A1), which we will touch upon here. This patent is for a varnish developed to form charge-transporting thin films with high charge-transporting properties even when annealed at lower temperatures. The patented varnish contains IBX and the Dess-Martin reagent (Dess-Martin periodinane: DMP) introduced in this article. Specifically, IBX and DMP are dissolved together with charge-transporting material using an organic solvent to prepare the varnish.
According to the patent, utilizing this varnish to form the OLED hole injection layer, even when annealed at lower temperatures, allows for a reduced drive voltage and improved luminance characteristics without sacrificing the current efficiency of the element. Furthermore, the thin film produced by this patented varnish has other anticipated applications, including in antistatic film and the hole collection layer of organic thin film solar cells.
Clearly, iodyl compounds hold the capacity to contribute toward the realization of thin and flexible OLEDs. There is much to look forward to regarding future applications of these compounds.
MANAC offers manufacturing and marketing services for hypervalent organoiodine compounds. In particular, as we succeeded in significantly lowering our manufacturing costs of (diacetoxyiodo)benzene (DAIB), we can provide high-quality products at low prices.
Please feel free to inquire at the email address below.
chemia@manac-inc.co.jp
References
1) MANAC Inc. Research Lab., Suzuki, H. (ed.). Shuuso oyobi Youso Kagoubutsu no Yuuki Gousei: Shiyaku to Gouseihou [Organic Syntheses of Bromine & Iodine Compounds: Reagents & Synthesis Methods], Maruzen Publishing (2017).
2) Sharefkin, J. G., Salzman, H. Org. Synth. Coll. Vol. V, 665 (1973).
3) Formo, M. W., Johnson, J. R. Org. Synth. Coll. Vol. III, 486 (1955).
4) Boeckman, R. K. Jr, Shao, P. et al. Org. Synth., 2000, 77, 141; Coll. Vol. X, 696 (2004).













