
Addition of bromine to alkenes, bromohydrination of alkenes, synthesis of bromoalkanes from alcohols: NBS bromination reactions (3): N-bromo compounds (5): Discussion series on bromination/iodination reactions 5
In this series, we discuss bromination and iodination reactions, specialties of MANAC. Various NBS bromination reactions are reviewed throughout several articles.
This article covers the addition of bromine to alkenes, the bromohydrination of alkenes, and the synthesis of bromoalkanes from alcohols. Using NBS enables bromine to be added to alkene double bonds, and can also substitute allylic or benzylic position alcohol groups with bromine. We will discuss these reaction mechanisms and provide examples. Be sure to use this information as a reference in your research and experiments.
■ What you can learn from this article ✔ NBS not only replaces hydrogen atoms in compounds with bromine atoms, but it can also add bromine atoms to compounds. ✔ By using NBS in bromine addition reactions to alkenes, it is possible to add a bromine atom to one of the carbon atoms in the double bond, while adding a substituent other than bromine (such as OH or OR) to the other carbon atom. ✔ If complex compounds are desired, bromination using NBS is recommended. ■ Recommended Articles ・ A-bromination of carbonyl groups, bromination of carboxylic acids and related compounds: NBS bromination reactions (4): N-bromo compounds (6): Discussion series on bromination/iodination reactions 6 ・ Active/inactive aromatic ring bromination: Bromination reactions that use NBS (2): N-bromo compounds (4): Discussion series on bromination/iodination reactions 4
contents
Describing N-bromosuccinimide (NBS)
A very commonly used, easy-to-handle brominating agent
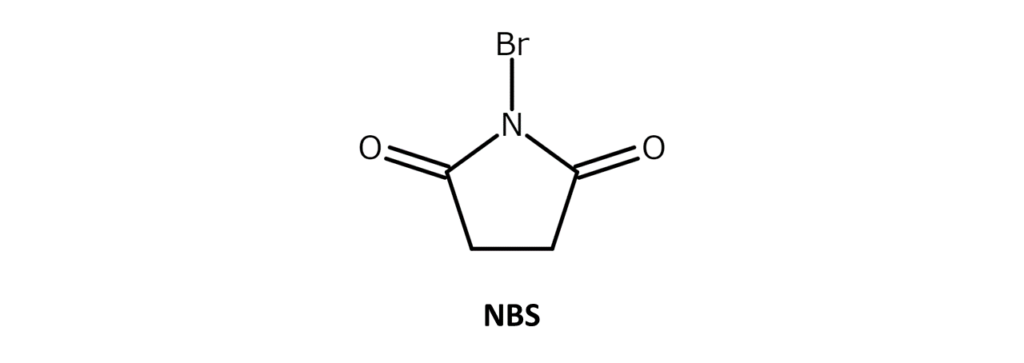
NBS is a white to slightly yellow crystalline powder with a weak bromine odor and a melting point of 173–176°C (decomposition). The compound is soluble in substances such as acetone, THF, DMF, acetonitrile, and DMSO, slightly soluble in water and acetic acid, and has poor solubility in hexane and carbon tetrachloride.
NBS powder is easier to handle than bromine (liquid), making it a common first-line brominating agent in organic syntheses. With its ability to undergo long-term storage in cool, dark, dry places, NBS is also relatively inexpensive.
This article covers information regarding the characteristics and precautions of NBS and an overview of bromination reactions that use NBS.
NBS bromination reactions: addition of bromine to alkenes, bromohydrination of alkenes
Reaction details
Thus far, we have discussed allylic position and benzylic position bromination as well as active/inactive aromatic ring bromination. NBS is used in these reactions to substitute certain atoms in compounds, such as hydrogen atoms, with bromine atoms. In addition to such substitutions, NBS can also be used to add bromine atoms.
The nitrogen atom in NBS is adjacent to an electron-withdrawing carbonyl group, making the bromine atom in NBS positively polarized and electrophilic toward electron-rich compounds. The addition of bromine to alkenes and the bromohydrination of alkenes being discussed in this issue are both reactions that involve the addition of NBS bromine atoms to alkene double bonds under the presence of Lewis acids or polar solvents (electrophilic addition reactions).
These reactions proceed by an ionic mechanism via a 3-membered ring bromonium ion intermediate with a positive bromine atom (structured with bromine bridging the two carbon atoms in the original double bond). After the bromonium ion forms from the NBS electrophilic attack, it is then attacked by another nucleophile (such as water or an alcohol), generating bromohydrin (if the nucleophile is water) or an ether (if the nucleophile is an alcohol).
Since the bromine atom in the bromonium ion is sterically large, the bromonium ion can only be nucleophilically attacked from the opposite side of the bromine atom. This usually makes it possible to gain adducts with a 1,2 trans arrangement.
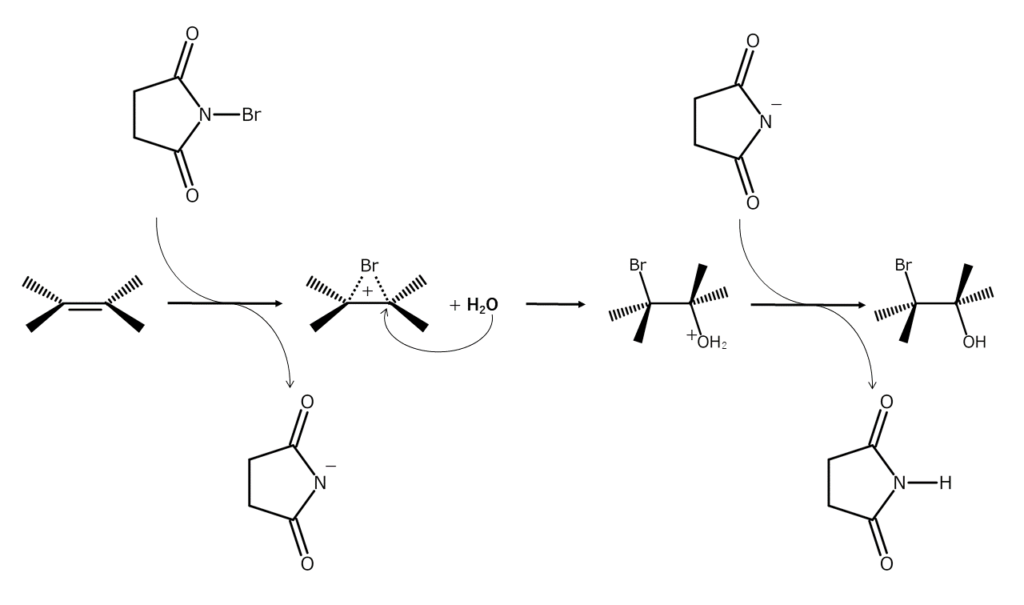
Addition reactions for simple alkenes can easily be carried out at low temperatures, but for (electron-deficient) alkenes with electron-withdrawing groups on unsaturated carbon, the addition of heat or catalysts is required. Since an addition-elimination reaction also occurs competitively, alkenes with brominated olefinic carbon may also occur together.
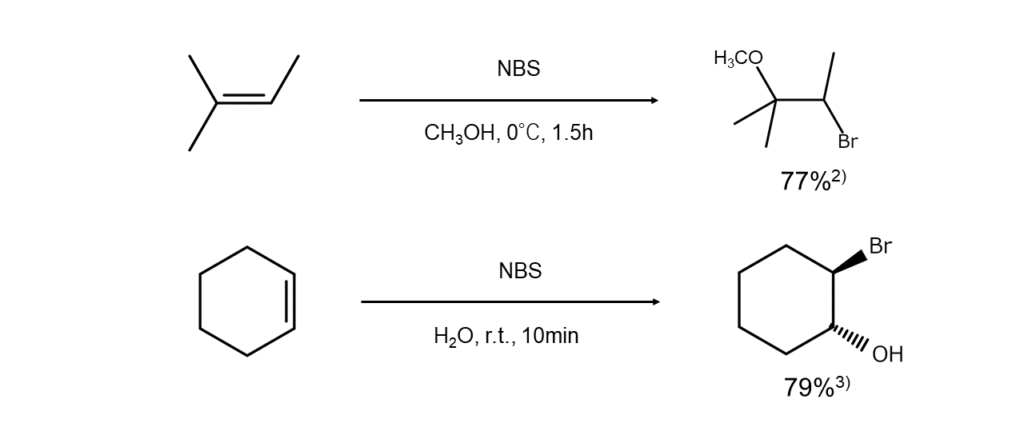
Examples of alkene bromination and bromohydrination reactions using NBS are shown below.
[MANAC Tidbits] Using NBS enables the generation of complex compounds
Using NBS for bromine addition reactions to alkenes allows for the addition of a bromine atom to one of the double-bond carbon atoms and a substituent other than bromine (such as OH or OR) to the other. As there are different substituents in each of the addition product molecules, these products can be derived into even more complex molecules.
Conversely, using bromine (Br2) instead of NBS as a brominating agent may result in products with bromine atoms added to both carbon atoms since the bromide ions generated from the bromine can also nucleophilically attack bromonium ions.
We recommend NBS bromination reactions when more complex molecules are required.
NBS bromination reactions: bromoalkane synthesis from alcohols
Reaction details
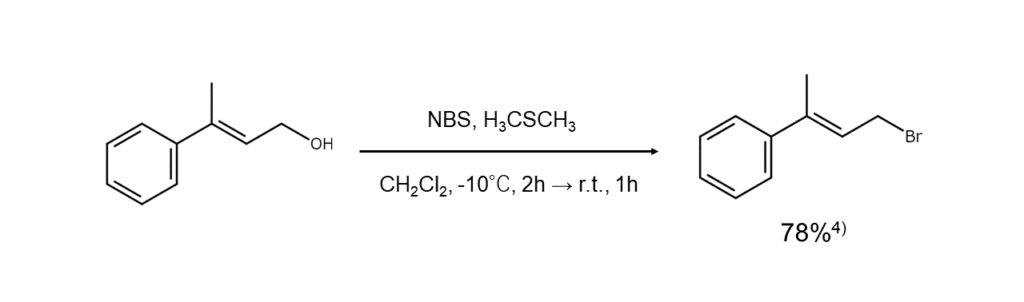
Combining NBS with dimethyl sulfide (DMS) results in sulfonium complexes, which are adducts of both. These sulfonium complexes can substitute allylic or benzylic position hydroxy groups (OH groups) with bromine atoms under mild conditions. Unsaturated bonds remain unaffected in this reaction.
The reaction mechanism is illustrated below.
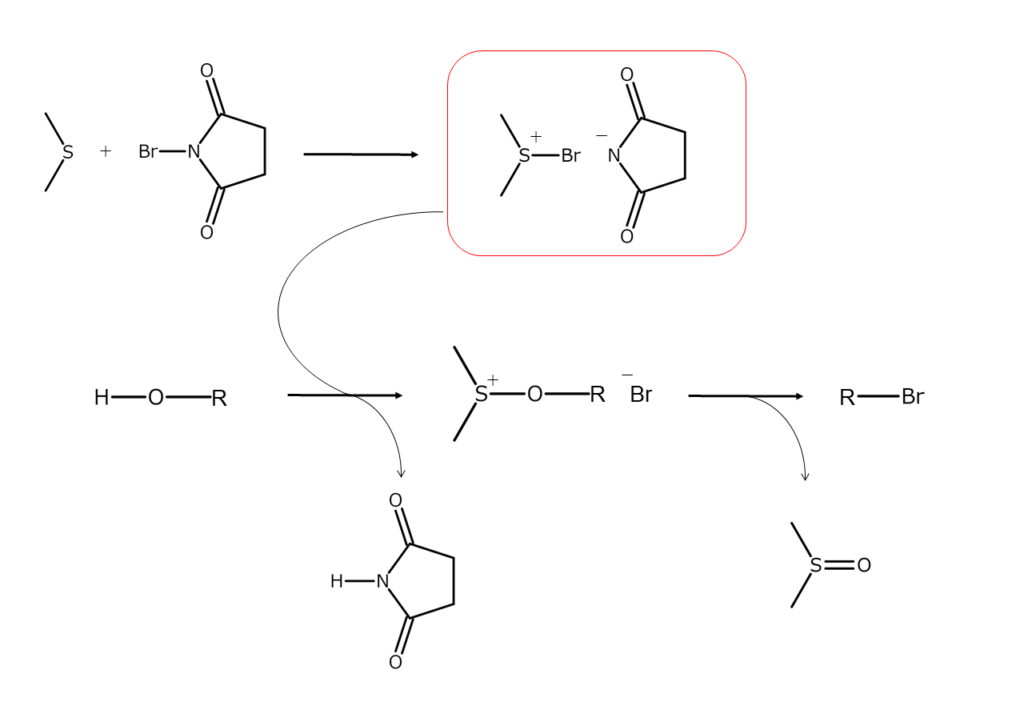
The example below illustrates the synthesis of bromoalkane from alcohols through the reaction mechanism above.
MANAC manufactures and sells NBS and DBDMH, two common N-bromo compounds. Please feel free to inquire.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing
2) Winstein, S., Ingraham, L. L. J. Am. Chem. Soc., 1952, 74, 1160.
3) Guss, C. O., Rosenthal, R. J. Am. Chem. Soc., 1955, 77, 2549.
4) Murphy, J. A., Patterson, C. W. J. Chem. Soc., Perkin Trans. 1 1993, 405.
















