
Bromination reactions with hydrogen bromide (Brominations via HBr-oxidant combinations and other syntheses with HBr): Hydrogen bromide (5): Discussion series on bromination/iodination reactions 38
In this series, we discuss bromination and iodination reactions, specialties of MANAC. We dedicated the previous four articles to various bromination reactions that use hydrogen bromide (HBr). These articles are intended to help readers understand the breadth of HBr applications, from bromoalkane synthesis to additions to alkenes and alkynes, as well as bromomethylation.
We conclude our coverage of HBr reactions with this article. Last but far from least in this coverage are bromination reactions via HBr-oxidant combinations, as well as other syntheses with HBr involving bromine atom rearrangements and substitutions.
Brominations with HBr-oxidant combinations leverage elemental bromine generated in situ as a brominating agent and offer the advantages of easy reaction management and low levels of side reactions. Other syntheses with HBr occur through rearrangement or substitution reactions using acid or heat, and are gaining attention as methods to produce compounds unobtainable with direct bromination. Both are crucial topics when learning about bromination reactions.
This time, we review the basic principles, characteristics, and other such details of these reactions. Be sure not to miss any details.
contents
Bromination reactions with hydrogen bromide: Brominations via HBr-oxidant combinations
Adjusting oxidant volumes to control reactions
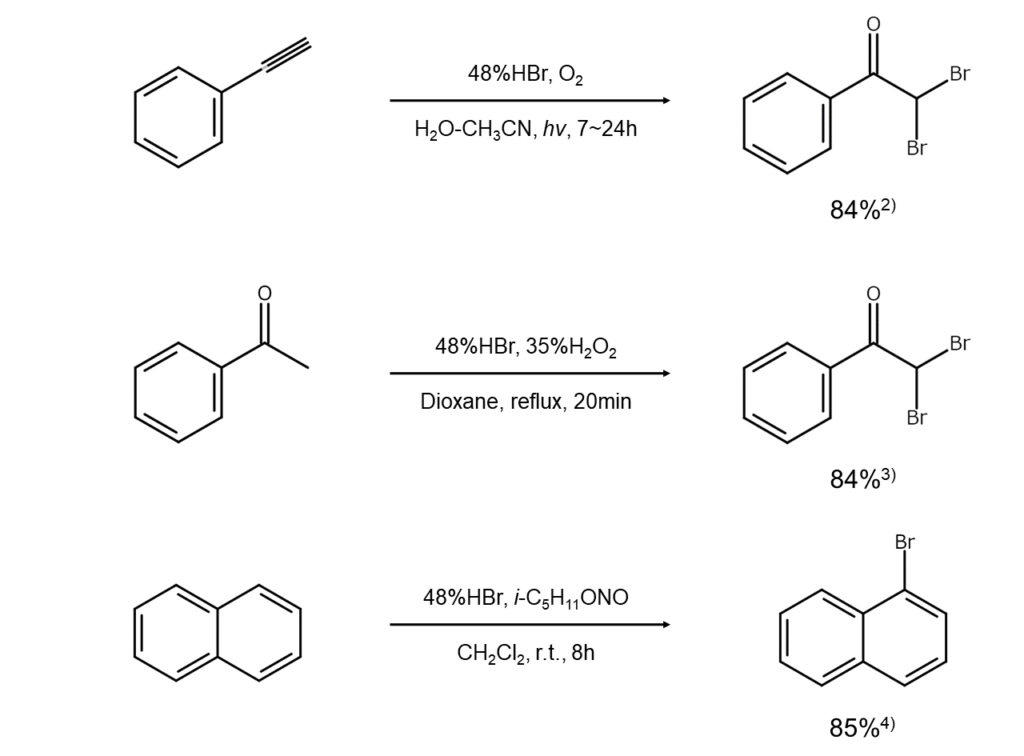
Alkenes, aromatic rings, and other such targets can be brominated by combining HBr with an appropriate oxidant to generate bromine (Br2) and using the bromine in situ as a brominating agent.
Since these reactions can be conducted while keeping bromine at exceptionally low concentration levels, they are often used to brominate activated substrates. Further, since the concentration levels of the bromine generated can be controlled with the oxidant volume ratio, better results with fewer side reactions can be achieved compared to directly reacting the target with elemental bromine.
The four reaction system types below are commonly used to brominate alkenes and aromatic rings and convert alkenes into bromohydrins.
HBr/DMSO system: (CH3)2SO + HBr → (CH3)2S + H2O + Br2
HBr/H2O2 system: H2O2 + 2HBr → H2O + Br2
HBr/O2(hν) system: O2 + 4HBr + hν → H2O + Br2
HBr/NaNO2/O2 system: 2NaNO2 + 4HBr → 2NOBr + 2NaBr + H2O
2NOBr → 2NO + Br2
The following are examples of bromination reactions that use HBr-oxidant combinations.
Bromination reactions with hydrogen bromide: Other syntheses with HBr
Bromine rearrangement reactions in aromatic bromine compounds
The C-Br bonds in bromine compounds are weak and easily cleaved compared to C-F and C-Cl bonds. As a result, the C-Br bonds are readily broken by the action of acids, heat, light, and other such factors. In many cases, the bromine atoms that occur are often observed to relocate somewhere either within or outside the molecule.
Bromine rearrangement reactions such as these may occur due to hydrogen bromide, the focus of this article. The example below illustrates hydrobromic acid reacting with an activated aromatic bromine compound, causing an intramolecular rearrangement reaction of the bromine atoms.

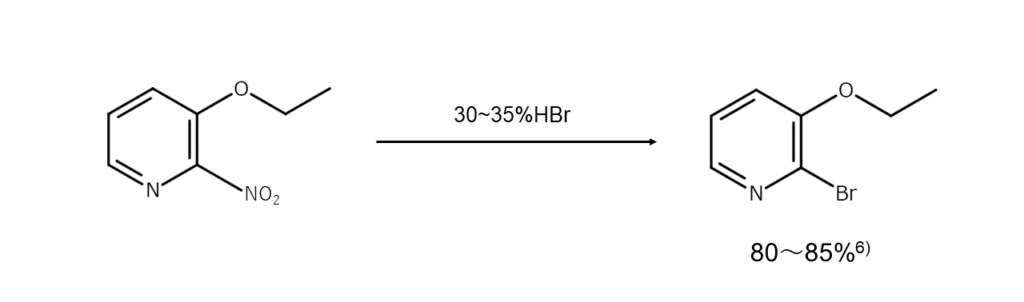
Reactions that substitute nitro groups in aromatic compounds with bromine atoms
The final reaction type introduces bromine atoms through a substitution reaction. Heating an aromatic compound containing nitro groups in the activated positions with concentrated hydrobromic acid for an extended period will enable the nitro groups to be substituted with bromine atoms.
Column: Unexpected synthesis methods for bromine compounds – The various rearrangement reactions of bromine atoms
So far in this article, we reviewed rearrangement reactions of bromine with hydrobromic acid as “other syntheses with HBr.” You may be surprised to learn that such rearrangement reactions can occur under other various conditions. Let’s take a look at a few of these rearrangement reactions that can be described as “special synthesis methods for bromine compounds.”
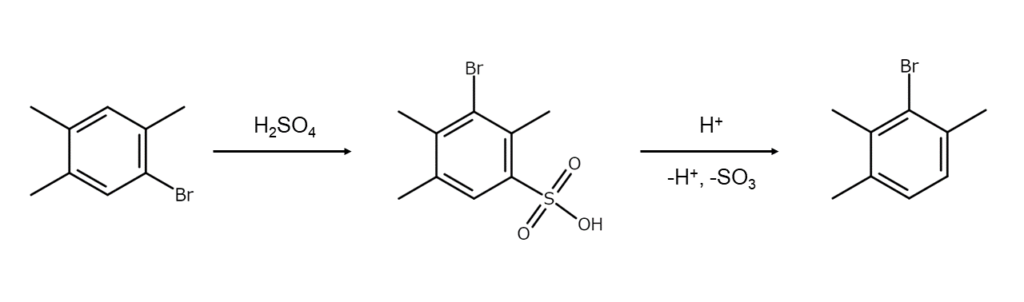
Methods with heat and concentrated sulfuric acid or Al2Cl6
Heating aromatic bromine compounds that lack a strong electron-withdrawing group with concentrated sulfuric acid or aluminum chloride (Al2Cl6) will often rearrange bromine atoms, generating isomers or polybromo compounds. The Jacobsen reaction, shown below, is a well-known example and is used when changing a bromine compound with a non-vicinal structure into an isomer with a vicinal structure.
Methods with only heat
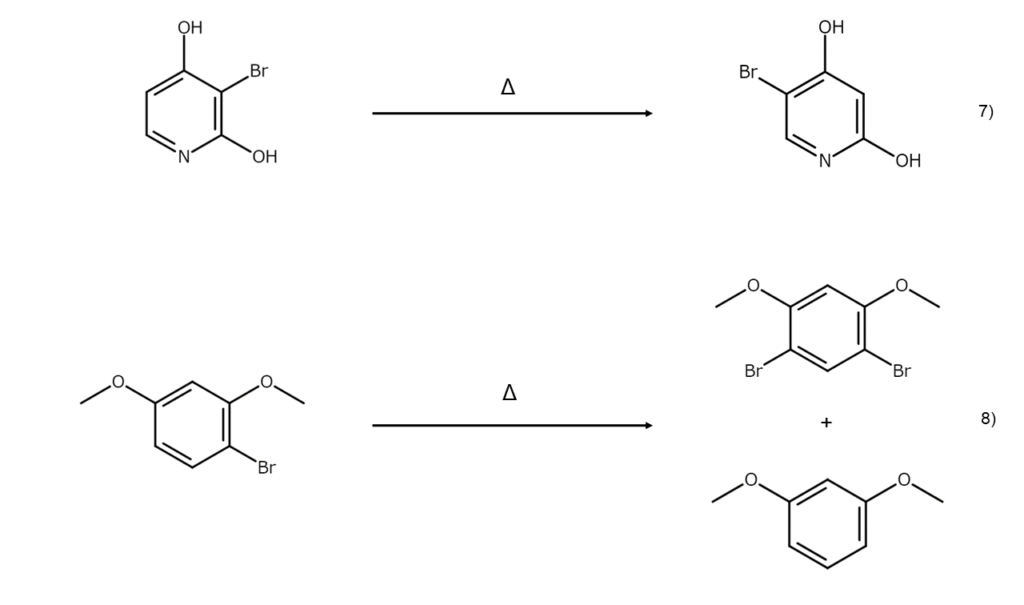
In some instances, rearrangement reactions will occur with heat alone, even without an acid. Phenols, resorcin, anilines, and other bromine derivatives are known to undergo isomerization or partial disproportionation solely through heating at high temperatures, causing them to readily change into a different bromine compound.
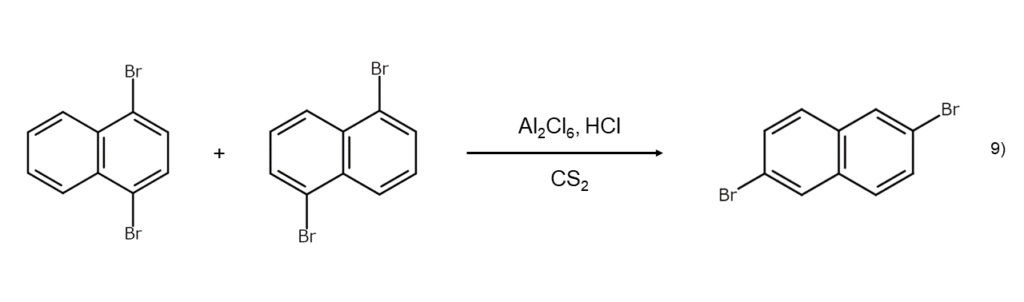
Methods using carbon disulfide with Al2Cl6 and HCl
The dibromination of naphthalene can yield a mixture of 1,4-dibromonaphthalene and 1,5-dibromonaphthalene. Dissolving and leaving this mixture in carbon disulfide with Al2Cl6 and hydrogen chloride (HCl) will cause both bromine atoms in the molecule to migrate, yielding 2,6-dibromonaphthalene. Such isomerizations catalyzed by Lewis acids are useful as synthesis methods to obtain compounds that are not possible through direct bromination.
MANAC is a global leader in bromination and iodination reactions. Please inquire with us about any bromination or iodination needs.
References
1) MANAC Inc. Research Lab., Suzuki, H. (ed.). Shuuso oyobi Youso Kagoubutsu no Yuuki Gousei: Shiyaku to Gouseihou [Organic Syntheses of Bromine & Iodine Compounds: Reagents & Synthesis Methods], Maruzen Publishing (2017).
2) Nobuta, T., Hirashima, S. et al. Tetrahedron Lett., 2010, 51, 4576.
3) Terent’ev, A. O., Khodykin, S. V. et al., Synthesis, 2006, 1087.
4) Gavara, L., Boisse, T. et al. Tetrahedron, 2008, 64, 4999.
5) Koenigs, Z., Gerdes, H. C. et al. Chem. Ber., 1928, 61, 1027.
6) den Hertog, H. J., Jouwersma, C. et al. Rec. trav. chim. Pays-Bas, 1949, 68, 275.
7) den Hertog, H. J., Schogt, J. C. M. et al. Rec. trav. chim. Pays-Bas, 1950, 69, 673; ibid., 1951, 70, 353.
8) Meerwein, H., Hofmann, P. et al. J. prakt. Chem., 1940, [2], 154, 266.
9) Salkind, J., Stetzuro, Z. Chem. Ber., 1931, 64, 953.













