
Applications as halogenating reagents – An overview of hypervalent organoiodine compounds and explanation of dihalogenoiodo compounds: Hypervalent organoiodine compounds (1): Discussion series on bromination/iodination reactions 29
Here on Chemia, we have discussed bromination and iodination reactions, specialties of MANAC. In this article, we begin our coverage of hypervalent organoiodine compounds.
Some may be unfamiliar with the concept of “hypervalent organoiodine compounds.” And you may be wondering, “What kind of properties do these compounds have?” Maybe you would like to know about the different applications of these compounds. Hypervalent organoiodine compounds certainly offer a multitude of excellent advantages. Throughout this and several upcoming articles, we will uncover the secrets of these compounds.
In this first article on the topic, we will start by giving an overview of hypervalent organoiodine compounds and explaining the characteristics and reactions of dihalogenoiodo compounds, which are a type of hypervalent organoiodine compounds. Be sure to enjoy the entire article.
contents
Hypervalent organoiodine compounds
Organic compounds containing hypervalent iodine
A hypervalent organoiodine compound is just that — an organoiodine compound that is hypervalent.
Atoms are “hypervalent” when the most outer electron shell (the part of the atom related to bonds with other atoms), which sits furthest from the nucleus, contains more than eight electrons. This state enables the atom to form a higher number of bonds with other atoms. For example, iodine atoms gain the ability to bond with multiple atoms at once when they become hypervalent, as shown in the figures below (the iodine atom on the left is trivalent, while the iodine atom on the right is pentavalent). When an organic compound contains hypervalent iodine, it is known as a “hypervalent organoiodine compound.”
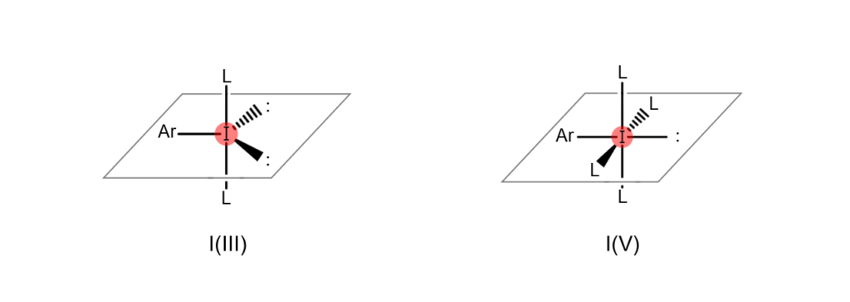
In the past, most organoiodine compounds obtained in the laboratory were monovalent. However, in recent years, many hypervalent compounds like those above are now being synthesized, among which some are available commercially as reagents.
Examples of well-known hypervalent organoiodine compounds include (dichloroiodo)benzene, (diacetoxyiodo)benzene, iodosylbenzene, and iodylbenzene.
The roles of hypervalent organoiodine compounds as oxidants, dehydrogenating agents, and alternative reagents to toxic elements
Hypervalent organoiodine compounds have played many roles in the laboratory, including as mild oxidants, dehydrogenating agents, and as reagents for introducing functional groups.2)
Additionally, these compounds often exhibit chemically similar behaviors to certain compounds like organomercury(II), organothallium(III), and organolead(IV) compounds, enabling hypervalent organoiodine compounds to replace these toxic elements as alternative reagents. Further, in recent years, iodonium salts such as polyfluoroalkyliodonium, alkynyliodonium, and diaryliodonium salts are relatively inexpensive and available commercially as alkylating or arylating agents that leverage the high elimination properties of hypervalent iodine.
Learning more about hypervalent organoiodine compounds
Starting with this article, we are dedicating several issues to discussing the characteristics and reactions of various hypervalent organoiodine compounds. However, for those who would like to learn more than what can be covered in a series of articles, we recommend consulting references 2–14 listed at the end, which include reference books and review articles.
Introducing hypervalent iodine compounds: Dihalogenoiodo compounds
Dichloroiodoarenes
Dichloroiodoarenes are yellow-orange crystalline solids. Although typically stable under normal handling conditions, dichloroiodoarenes rapidly degrade when heated or exposed to sunlight. These compounds are also unsuited for long-term storage as they steadily degrade even in cool, dark conditions.
A known method for synthesizing dichloroiodoarenes reacts an iodoarene with chlorine in chloroform, oxidizing the iodine atoms.15) Other methods react an iodoarene with an acid under the presence of an oxidant, such as sodium perborate,16) sodium persulfate,17) potassium chlorate,18) or chromium oxide.19) Reaction examples are shown below.
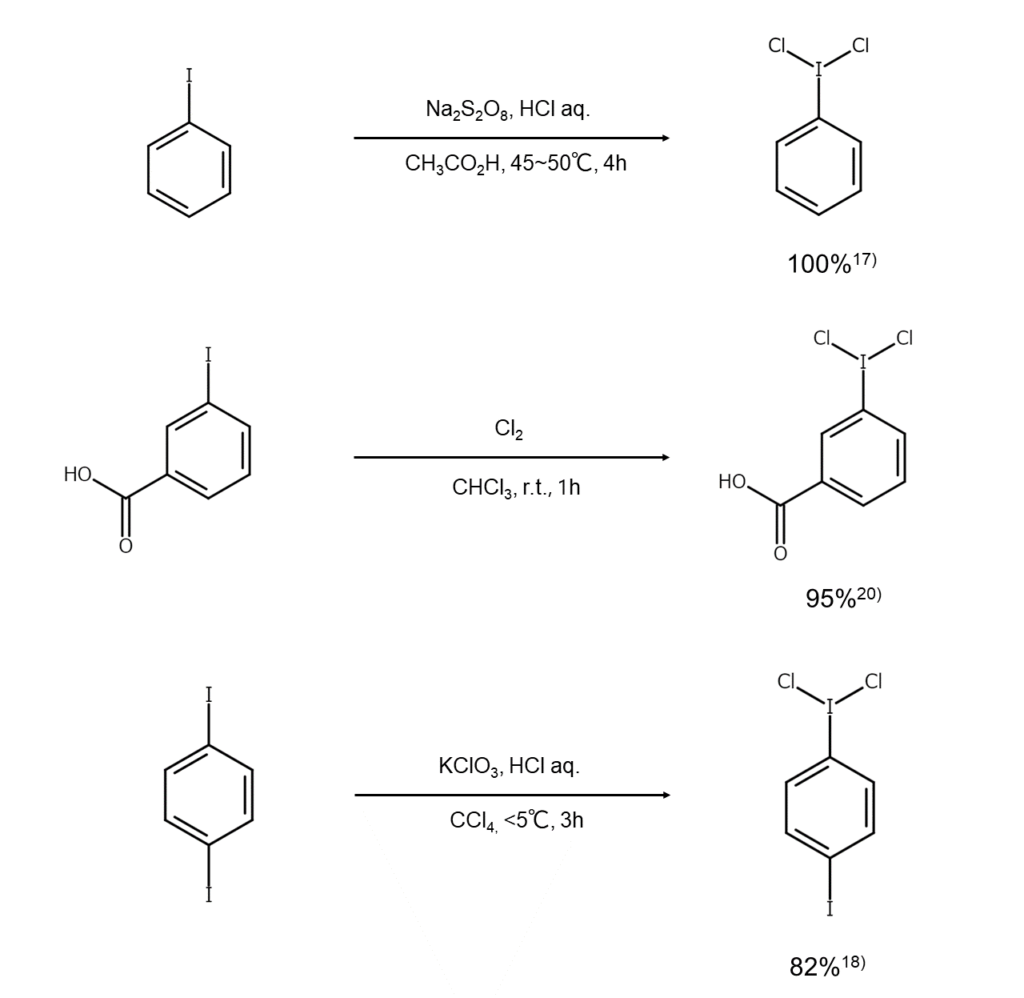
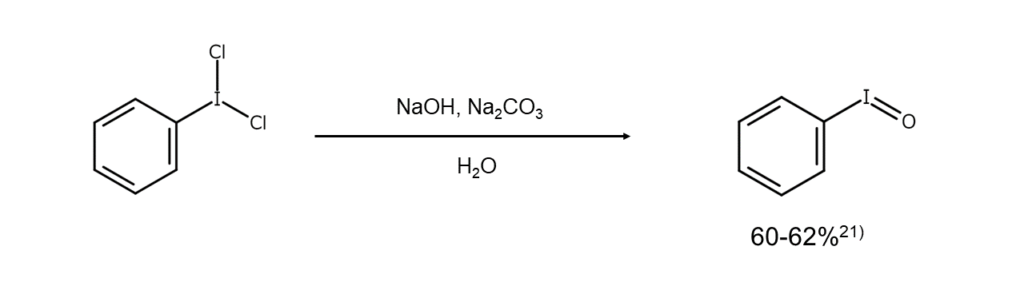
Dichloroarenes are used in laboratories as mild chlorinating agents. When a dichloroarene is used in a chlorine addition reaction for an alkene, trans bodies are obtained under ionic reaction conditions, while a mixture of cis/trans bodies are gained under radical reaction conditions. Also, dichloroarenes are converted into an iodosylarene when subjected to hydrolysis with a dilute NaOH aqueous solution.21)
Difluoroiodoarenes
Difluoroiodoarenes are compounds used in the fluorination of alkenes, carbonyl compounds, iodoalkanes, and other such compounds.
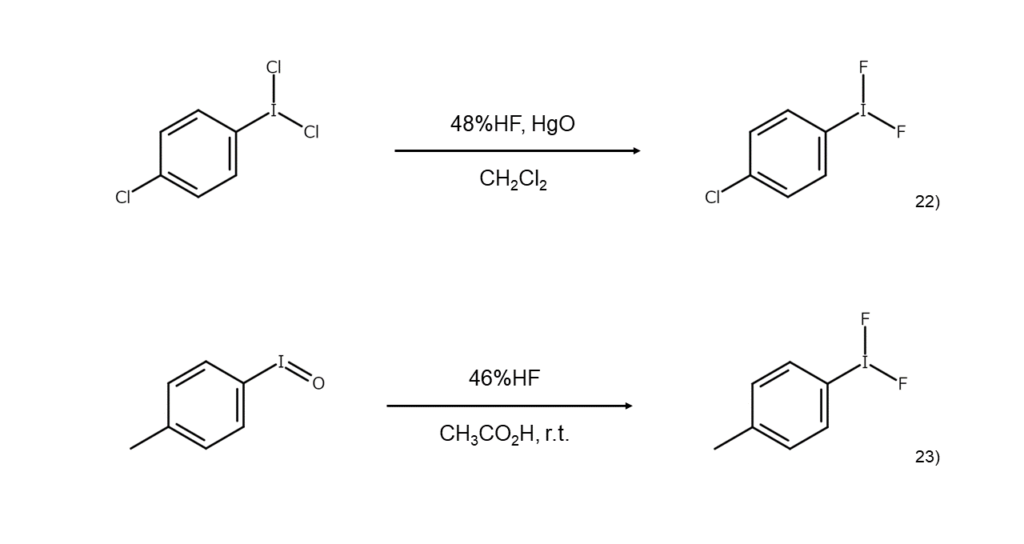
Known synthesis methods for difluoroiodoarenes include reacting a dichloroiodoarene with hydrofluoric acid under the presence of HgO,22) treating an iodosylarene with hydrofluoric acid23) or SF4,24) and electrochemically oxidizing an iodoarene under the presence of triethylamine trihydrofluoride ((C2H5)3N·3HF).25)
Column: The story behind hypervalent iodine compound nomenclatures
The trivalent and pentavalent iodine compounds introduced in this article are customarily named as derivatives of hypothetical hydrides iodinane (H3I) and periodinane (H5I). However, the International Union of Pure and Applied Chemistry (IUPAC) recommends its new nomenclature, which posits hydrogen iodide (HI) as the reference compound named “iodane.”
IUPAC nomenclature denotes compounds containing bonds exceeding standard bonding numbers with the lambda symbol “λ” superscripted with the number of bonds. Following this convention, the “iodinane” above becomes “λ3-iodane” while “periodinane” becomes “λ5-iodane.”
Regardless of IUPAC recommendations, this new nomenclature remains largely unadopted, including by researchers in this field. For this reason, we are using the common and conventional names in this article.
Nevertheless, those who gain an understanding of the new naming convention will be better equipped to examine a broader range of references and even appropriately select between the nomenclatures as needed in different applications. When possible, learning about both conventional and new nomenclatures should prove advantageous.
MANAC offers manufacturing and marketing services for hypervalent organoiodine compounds. In particular, as we succeeded in greatly lowering our manufacturing costs of (diacetoxyiodo)benzene (DAIB), we can provide high-quality products at a low cost.
Please feel free to inquire at the email address below.
chemia@manac-inc.co.jp
References
1) MANAC Inc. Research Lab., Suzuki, H. (ed.). Shuuso oyobi Youso Kagoubutsu no Yuuki Gousei: Shiyaku to Gouseihou [Organic Syntheses of Bromine & Iodine Compounds: Reagents & Synthesis Methods], Maruzen Publishing (2017).
2) Willgerodt, C. Die organischer Verbindungen mit mehrwertigen Jod, Ferdinand Enke (1914).
3) Sandin, R. B. Chem. Rev., 1943, 32, 249.
4) Beringer, F. M., Gindler, E. M. Iodine Abstract Rev., 1956, 3, 70.
5) Banks, D. F. Chem. Rev., 1966, 66, 243.
6) Varvoglis, A. Chem. Soc. Rev., 1981, 10, 377.
7) Varvoglis, A. Hypervalent Iodine Chemistry (Top. Curr. Chem. 224), Springer (2003).
8) Merkushev, E. B., Schwarzberg, M. C. Organoiodine Compounds in Organic Synthesis, Tomsk Ped. Inst. Tomsk, USSR (1978).
9) Varvoglis, A. Synthesis, 1984, 709.
10) Varvoglis, A. The Organic Chemistry of Polycoordinated Iodine VCH, New York (1992).
11) Wirth, T., Hirt, U. H. Synthesis, 1999, 1271.
12) Stang, P. J., Zhdankin, V. V. Chem. Rev., 1996, 96, 1123.
13) Zhdankin, V. V., Stang. P. J. Chem. Rev., 2002, 102, 2523.
14) Zhdankin, V. V., Stang. P. J. Chem. Rev., 2008, 108, 5299.
15) Lucas, H. J., Kennedy, E. R. Org. Synth. Coll. Vol. III, 482 (1955).
16) Koyuncu, D., McKillop, A. et al. J. Chem. Res. (S), 1990, 21.
17) Baranowski, A., Plachta, D. et al. J. Chem. Res. (S), 2000, 435.
18) Krassowska-Swiebocka, B., Prokopienko, G. et al. Synlett, 1999, 1409.
19) Kazmierczak, P., Skulski, L. J. Chem. Res. (S), 1999, 64.
20) Yusubov, M. S., Drygunova, L. A. et al. Synthesis, 2004, 2289.
21) Lucas, H. J., Kennedy, E. R. Org. Synth. Coll. Vol. III, 483 (1955).
22) Carpenter, W. J. Org. Chem., 1966, 31, 2688.
23) Garvey, Jr., B. S., Halley, L. F. et al. J. Am. Soc. Chem., 1937, 59, 1827.
24) Lyalin, V. V., Orda, V. V. et al. Zn. Org. Khim., 1970, 6, 329.
25) a) Fuchigami, T., Fujita, T. J. Org. Chem., 1994, 59, 7190. b) Fujita, T., Fuchigami, T. Tetrahedron Lett., 1996, 37, 4725.













