
Acyl iodide synthesis, iodoarene synthesis: Iodination reactions with halogen exchange (3): Discussion series on bromination/iodination reactions 14
While the iodides used as intermediates for electronic materials and pharmaceuticals are not in the final products, they help support our lifestyles from behind the scenes.
An essential synthesis method for iodides is the halogen exchange. The halogen exchange is a valuable method capable of iodinating a relatively large number of substrates and has long been used for various applications. Knowledge of halogen exchanges can surely come in handy when designing iodination reactions.
Our last article covered iodoalkane synthesis reactions, which can be described as synonymous with halogen exchange iodination. Continuing our review, this article covers halogen exchanges in acyl iodide synthesis and iodoarene synthesis reactions. As we discuss details of reaction mechanisms and example reactions, this information can be used as a reference for experiments.
■ What you can learn from this article ✔ The Finkelstein reaction can also be applied to acyl halides. ✔ It is possible to synthesize iodoarenes from bromoarenes and chloroarenes, but reaction conditions vary depending on the position and properties of the substituent on the aromatic ring. ✔ Because hypervalent iodine compounds have low toxicity and are environmentally friendly, they are used as oxidants in the synthesis of active pharmaceutical ingredients (APIs). ■ Recommended Articles ・ Iodoalkane synthesis: Iodination reactions with halogen exchange (2): Discussion series on bromination/iodination reactions 13 ・ Applications as halogenating reagents – An overview of hypervalent organoiodine compounds and explanation of dihalogenoiodo compounds: Hypervalent organoiodine compounds (1): Discussion series on bromination/iodination reactions 29 ・ (Diacyloxyiodo)arenes as effective gentle oxidants: Hypervalent organoiodine compounds (2): Discussion series on bromination/iodination reactions 30
contents
Describing the halogen exchange
A method for converting chlorides and bromides into iodides
The halogen exchange is used as a method to replace a halogen atom in a compound with a different halogen atom. In this case, the halogen exchange replaces a chlorine atom or a bromine atom with an iodine atom.
There are several types of halogen exchange reactions. Among these, the halogen exchange reaction most commonly used for iodination reactions is the Finkelstein reaction2, which replaces a halogen atom with a different halogen atom through a bimolecular nucleophilic substitution reaction (SN2 reaction). The reaction formula is shown below.
R-X + NaI ⇌ R-I + NaX X = Cl, Br
Because of its wide-ranging substrate applications, the Finkelstein reaction has long been used to synthesize iodoalkanes and other substances. See this article for details on Finkelstein reaction principles.
Halogen exchange iodination reactions: Acyl iodide synthesis
Reaction details
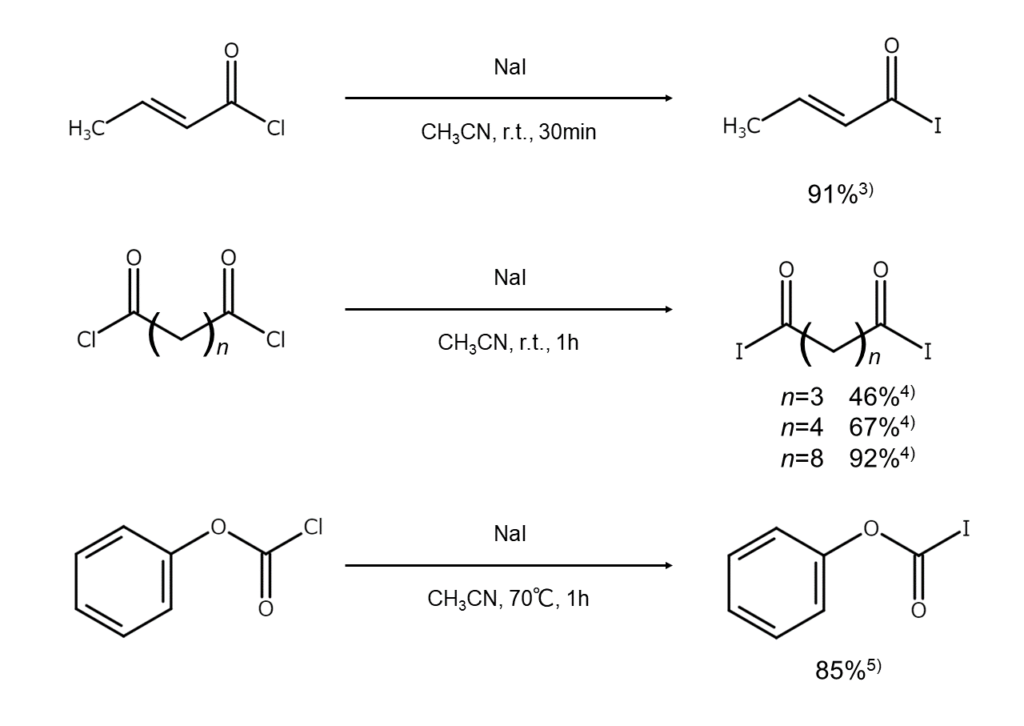
Finkelstein reactions can also be used for acyl halides. For example, setting NaI to act on acyl chloride in anhydrous acetonitrile allows for a high yield of the corresponding acyl iodide3-5. Unlike the iodoalkane synthesis explained in the previous article, these reactions can also be used to carry out halogen exchange for tertiary acyl halides without issue.
Since many acyl iodides are unstable, any portions that will not be used immediately should have a small amount of copper powder added and should undergo storage in a cool, dark place.
The following are examples of acyl iodide synthesis using alkali iodides.
Halogen exchange iodination reactions: Iodoarene synthesis
Reaction details
Using halogen exchange allows for the synthesis of bromoarenes, chloroarenes, and iodoarenes. However, reaction conditions differ depending on the position and properties of the substituent present on the aromatic ring. Details are as follows below.
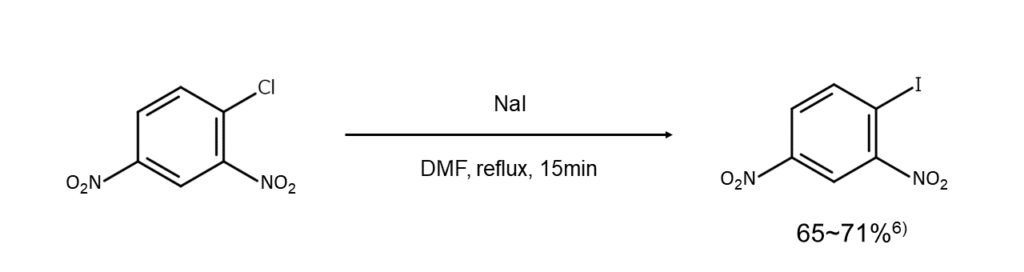
(1) With strong electron-withdrawing groups
The Finkelstein reaction (aromatic nucleophilic substitution reaction), in which the halogen substituent becomes the leaving group, can easily occur when a strong electron-withdrawing group (e.g., nitro group, carbonyl group, cyano group, etc.) is present in the ortho or para position of a halogen substituent.
For example, as shown below, heating 4-chloro-1,3-dinitrobenzene with sodium iodide in a polar solvent can yield the corresponding iodoarene.
Under the following conditions, a halogen exchange will also occur with pyridazine rings, of which the electron density will drop with the effects of the nitrogen atom.

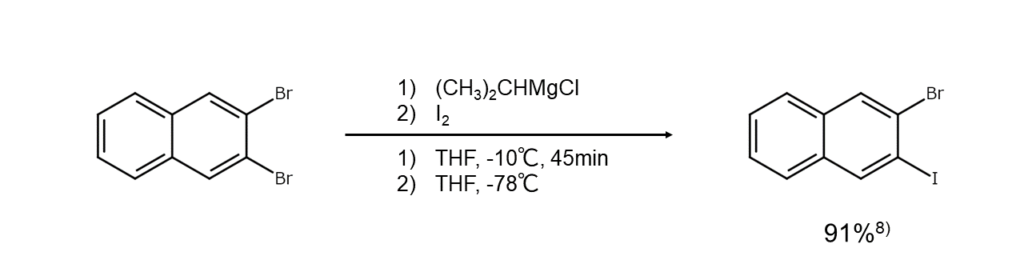
(2) With electron-donating groups
Chlorine and bromine derivatives with an electron-donating group, such as alkylbenzenes and phenol ethers, do not readily undergo Finkelstein reactions as the electron density on the aromatic ring increases.
A known solution involves a method in which the reactants are briefly changed into a Grignard reagent or an organic lithium compound and then treated with iodine (see the reaction formula below). In the case of polyhaloarenes, since the halogen atom in the activation position is substituted preferentially by Mg, this method can be useful for synthesizing iodoarenes of varied substitution arrangements depending on the execution. There is also a method that heats reactants with the Grignard reagent prepared from iodoarene to achieve a halogen-iodine exchange between them.
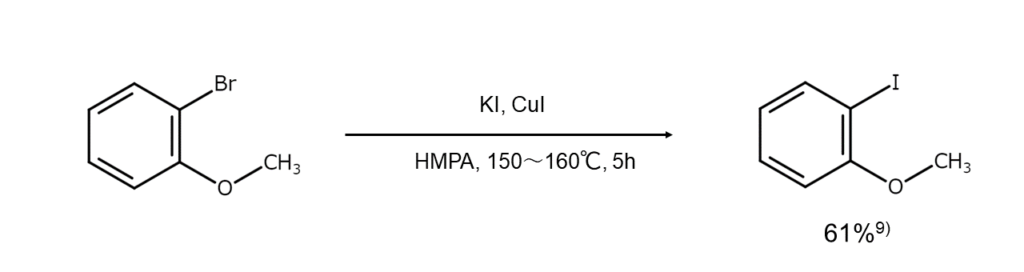
When the reactant is a bromoarene, effective methods include heating the reactant with an alkali iodide under the presence of CuI in a polar solvent with excellent coordination properties such as DMF or HMPA (see the reaction formula below) or reacting the reactant with an alkali iodide in the presence of nickel (II) salt. However, these reactions do not work well with chloroarenes.
Iodoarenes contribute to pharmaceutical manufacturing
Iodoarenes can be synthesized by leveraging halogen exchange reactions. But did you know about the deep connection between iodoarenes and pharmaceuticals?
Iodoarenes serve as starting materials when synthesizing hypervalent iodine compounds10 (compounds containing an iodine atom with a valence higher than 1). Hypervalent iodine compounds are incredibly beneficial substances with many advantages, including excellent stability, low volatility, high reactivity, and nearly zero toxicity. These compounds are used extensively in pharmaceuticals, fine chemicals, and other fields.
For example, hypervalent iodine compounds are used as oxidants when synthesizing active pharmaceutical ingredients (APIs). In contrast to traditional heavy-metal-based oxidants, oxidants based on hypervalent iodine compounds have low toxicity and are environmentally friendly. This makes them highly suited for synthesizing compounds that require a high level of safety, such as APIs.
In this way, iodoarenes play an essential role in supporting our daily health through the manufacturing of hypervalent iodine compounds.
MANAC markets DAIB, which has the simplest structure among hypervalent iodine compounds. Using our own original manufacturing methods, we have overcome many challenges, such as high manufacturing costs and the occurrence of byproducts, in order to provide high-quality products at a low cost. See the article below for details and the story behind the development of our manufacturing processes for DAIB.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing. (Japanese)
2) Finkelstein, H. Ber. 1910, 43, 1528.
3) Hoffmann, H. M. R., Haase, K. Synthesis, 1981, 715.
4) Hoffmann, H. M. R., Haase, K. et al. Synthesis, 1982, 237.
5) Hoffmann, H. M. R., Iranshahi, L. J. Org. Chem., 1984, 49, 1174.
6) Bunnett, J. F., Conner, R. M. J. Org. Chem., 1958, 23, 305; Org. Synth. Coll. Vol. V, 478 (1973).
7) Coad, P., Coad, R. A. et al. J. Org. Chem., 1963, 28, 218.
8) Cottet, F., Castagnetti, E. et al. Synthesis, 2005, 798.
9) Suzuki, H., Kondo, A. et al. Chem. Lett., 1985, 411.
10) Watanabe, A., Miyamoto, K. et al. J. Org. Chem., 2018, 83, 14262.

















