Alkyne iodination: Aliphatic compound iodination reactions (3): Discussion series on bromination/iodination reactions 20
Elemental iodine is easier to handle than other halogens since it exists as a solid at room temperature. This quality lends to the common usage of elemental iodine as an iodinating agent.
For this article, we dive into alkyne iodination reactions that use elemental iodine.
There are two approaches to alkyne iodination. The first approach adds iodine atoms to intramolecular acetylene bonds, while the second approach substitutes the hydrogen atom bonded to the terminal alkyne carbon with an iodine atom. Since reactions readily occur with either approach, knowledge of these reactions may be immensely helpful when synthesizing iodides.
As in our previous articles, this article provides details on reaction mechanisms and example reactions. Be sure to use this information as a reference.
■ What you can learn from this article ✔ The addition reaction of iodine to the triple bond of alkynes proceeds more smoothly compared to alkenes, and the resulting (E)-1,2-diiodoalkenes are stable and easy to isolate. ✔ For terminal alkyne carbon iodination, the choice of appropriate conditions and reagents, such as iodine-morpholine complexes or organometallic reagents, depends on the substrate. ✔ While iodinated alkyne products are increasingly applied as radioactive probes in the medical field, careful attention to reaction conditions and storage methods is essential. ■ Recommended Articles ・ Carbonyl compound iodination: Aliphatic compound iodination reactions (4): Discussion series on bromination/iodination reactions 21 ・ Iodine addition to alkenes: Aliphatic compound iodination reactions (2): Discussion series on bromination/iodination reactions 19
contents
Aliphatic compound iodination reactions with elemental iodine: Alkyne iodination
①Iodine addition to acetylene bonds
Smooth reactions and readily extractable products
The addition of elemental iodine to alkyne triple bonds occurs much more smoothly than the same addition to alkene double bonds. As with alkenes, this addition reaction proceeds through an anti-addition mechanism via an iodonium ion intermediate.
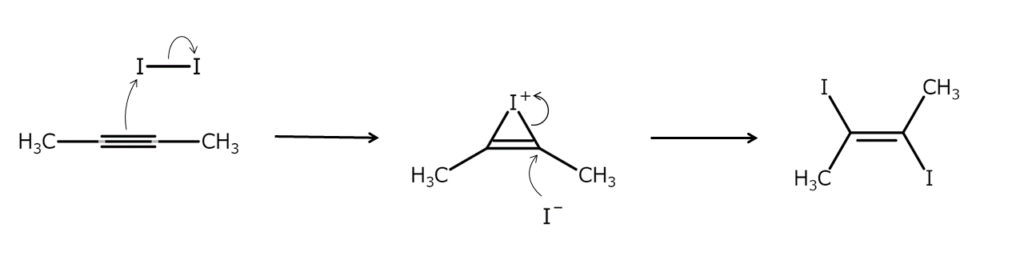
In addition to elemental iodine, alkyne iodine addition reactions are often carried out using iodine dissolved in a potassium iodide (KI) solution, as well as iodine generated in situ from alkali iodide and copper sulfate.
Many (E)-1,2-diiodoalkene reaction products are relatively stable and, therefore, readily extractable. However, these products are highly susceptible to E/Z isomerization with light exposure or through the action of minimal amounts of elemental iodine. Care should be taken in this regard.
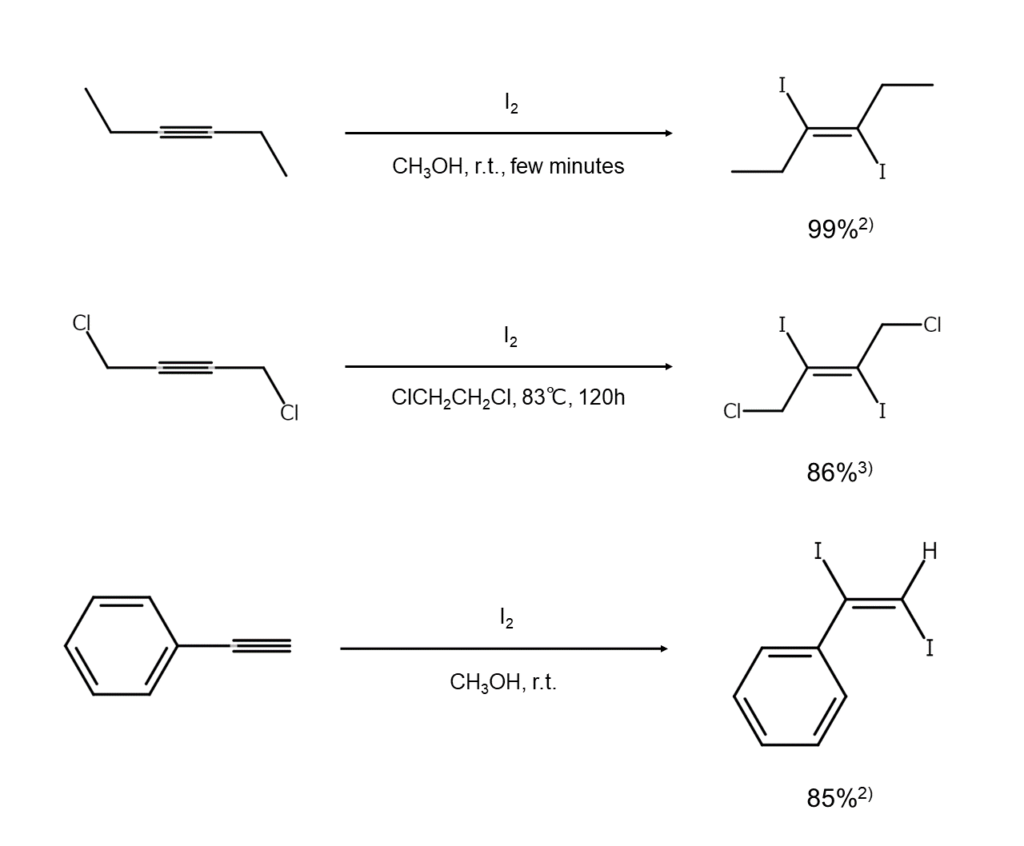
The following are examples of iodine addition reactions for alkynes.
Heterocyclic compound synthesis using compounds like alcohols and thiols
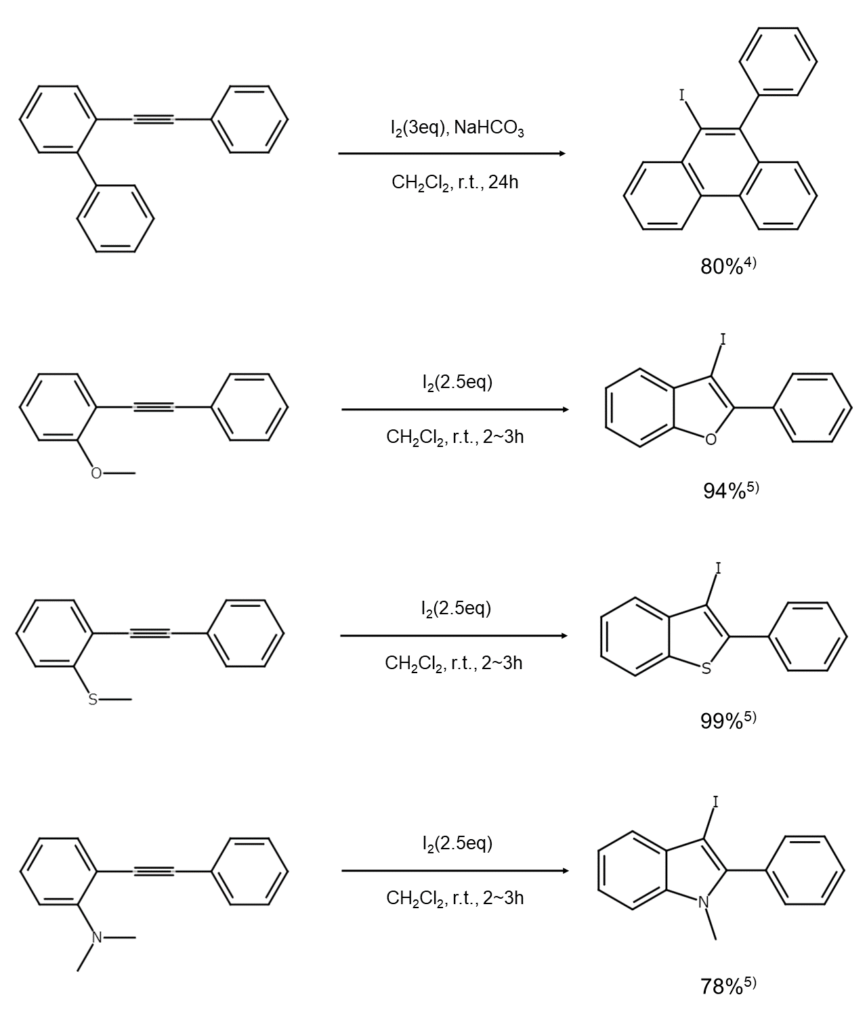
Compounds that have an acetylene bond in the molecule, such as alcohols, thiols, enones, and carboxylic acids, and are reacted similarly to the above will produce an iodonium ion intermediate from the iodination of unsaturated bonds. The intermediate undergoes an SN2 attack from an intramolecular heteroelement functional group (such as a hydroxy group), resulting in a high yield of the corresponding cyclic ether, sulfide, or ester.
In these types of reactions, complex skeletons can be created in a single step by effectively leveraging the substrate structure. For this reason, these reactions are widely used to synthesize heterocyclic compounds related to pharmaceuticals and natural products. The following are examples of common reactions (to learn more about related reactions, see references 6–10 listed at the end of this article).
②Terminal alkyne carbon iodination
Alkynes activated through conjugation: Relatively simple iodination
When 1-alkynes activated through conjugation with unsaturated bonds, such as those in arylacetylenes and vinylacetylenes, are processed with a morpholine-iodine complex or set to act with iodine in liquid ammonia, the terminal alkyne carbon will be iodinated with relative ease. The following is an example of this reaction.
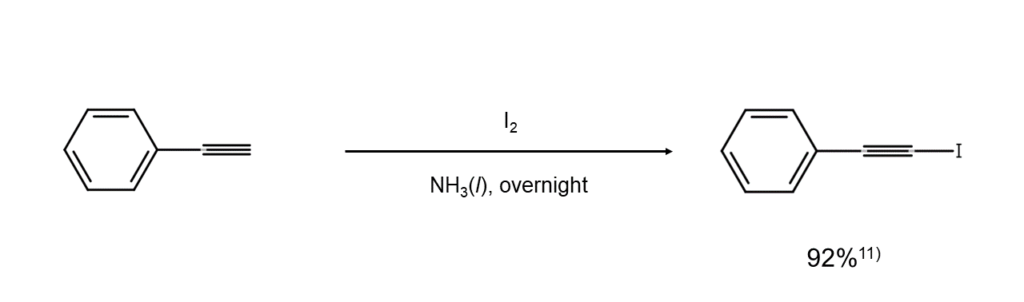
Further, if KI or t-butyl hydroperoxide is used, the above reaction can also be conducted under moderate conditions12.
Alkylacetylenes: Organometallic reagents required
Alkylacetylenes cannot be iodinated with the same methods used for arylacetylenes or vinylacetylenes. Instead, they are first converted into a metal acetylide using organic lithium, a Grignard reagent, or metallic sodium and then set to react with iodine to iodinate the terminal carbon. The following is an example reaction.
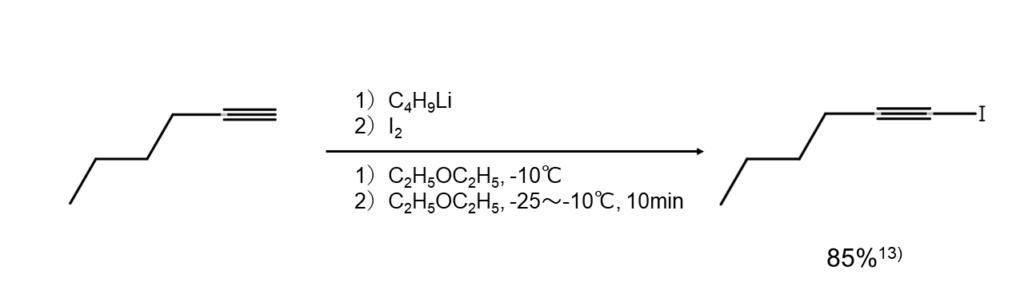
Reaction precautions
Since there is a strong tendency for 1-alkynes with iodinated terminal carbons to move toward conversion into a polyiodoalkene with further iodine addition, there must not be an excessive amount of iodine used during the reaction. As an effective way to avoid using excessive iodine, it has been proposed to have sodium hypochlorite (NaOCI) act on KI within the reaction system to generate hypoiodous acid (HIO), which would iodinate the alkyne carbon.
Additionally, iodoacetylenes and iododiacetylenes are both high-energy compounds, meaning there is a risk of explosion in a dry state. Be sure to exercise sufficient caution concerning storage methods.
The Breakroom Column: The surprising utility of (iodoethynyl)benzene as a radioactive probe
Let’s look back at the terminal alkyne carbon iodination reaction covered in this article from a slightly different angle. This reaction synthesizes (iodoethynyl)benzene, which may play a role in disease detection in the near future.

Healthcare frontlines currently conduct diagnostic imaging and radiotherapy based on radioactive probes, which are compounds labeled by radioisotopes. Compounds that contain radioactive iodine are effective radioactive probes.
Traditionally, aryl iodides and alkenyl iodides have been the radioactive probes of choice. However, Ferris et al.14 demonstrated that (iodoethynyl)benzene, iodinated at the terminal alkyne carbon, might be superior to iodophenol as a radioactive imaging probe owing to higher metabolic stability. Ferris et al. are reportedly working to further advance research toward practical applications.
Such developments illustrate how terminal alkyne carbon iodination reactions are being used in a wide range of fields.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing. (Japanese)
2) Heasley, V. L., Shellhamer, D. F. J. Org. Chem., 1980, 45, 4649.
3) Bridges, A. J., Fischer, J. W. Tetrahedron Lett., 1983, 24, 445.
4) Yao, T., Campo, M. A. et al. J. Org. Chem., 2005, 70, 3511.
5) Yao, T., Yue, D. et al. J. Org. Chem., 2005, 70, 10292; ibid. 2006, 71, 62.
6) Pacote, C. G., de Carvalho, B. S. et al. Synthesis, 2009, 3963.
7) Dowle, M. D., Davies, D. I. Chem. Soc. Rev., 1979, 8, 171.
8) Cardillo, G., Orena, M. Tetrahedron, 1990, 46, 3321.
9) Robin, S., Rousseau, G. Tetrahedron, 1998, 54, 13681.
10) Ranganathan, S., Muraleedharan, N. K. et al. Tetrahedron, 2004, 60, 5273.
11) Vaughan, T. H., Nieuwland, J. A. J. Am. Chem. Soc., 1933, 55, 2150.
12) Reddy, K. R., Venkateshwar, M. et al. Tetrahedron Lett., 2010, 51, 2170.
13) Kloster-Jensen, E. Tetrahedron, 1966, 22, 965.
14) Ferris, T., Carroll, L. et al. Tetrahedron Lett., 2019, 60, 936.









