
Carbonyl compound iodination: Aliphatic compound iodination reactions (4): Discussion series on bromination/iodination reactions 21
Over several issues in this series, we have covered alkane, alkene, and alkyne iodination reactions using elemental iodine. The nature of the reactions varies, ranging from those that do not readily undergo direct iodination to those that progress slowly, and others that proceed fairly smoothly.
In this article, we continue to look into carbonyl compound iodination reactions using elemental iodine.
While a number of issues plagued carbonyl compound iodination reactions, many improved methods have arisen in recent years to better facilitate such reactions. Learning about these improved methods will undoubtedly make it easier to design iodination reactions.
Be sure to enjoy this article in its entirety as we discuss the problems of traditional methods and explain the details of improved methods.
■ What you can learn from this article ✔ Traditional methods using elemental iodine have drawbacks, such as low yields and the formation of condensates. As a result, alternative methods, including the use of NIS, halogen exchange, and other improvements, have been proposed. ✔ NIS is an excellent iodinating agent due to its high reactivity. However, it is expensive because iodine is used as its raw material, making it unsuitable for large-scale synthesis. ✔ NIS exhibits high reactivity but cannot iodinate allylic positions. In contrast, NBS is cost-effective and can brominate allylic positions, making it necessary to choose the appropriate agent depending on the application. ■ Recommended Articles ・ Iodination of carboxylic acid and related compounds: Aliphatic compound iodination reactions (5): Discussion series on bromination/iodination reactions 22 ・ Alkyne iodination: Aliphatic compound iodination reactions (3): Discussion series on bromination/iodination reactions 20
contents
Aliphatic compound iodination reactions with elemental iodine: Carbonyl compound iodination
Traditional methods: Issues from low yields to steric hindrance sensitivity
Elemental iodine can be used to substitute the α-hydrogen in carbonyl compounds with iodine atoms. This is the carbonyl compound iodination reaction that we are discussing in this article.
The iodination of ketones with elemental iodine is generally conducted by generating enolate anions under the presence of a base. The reaction mechanism is shown below.
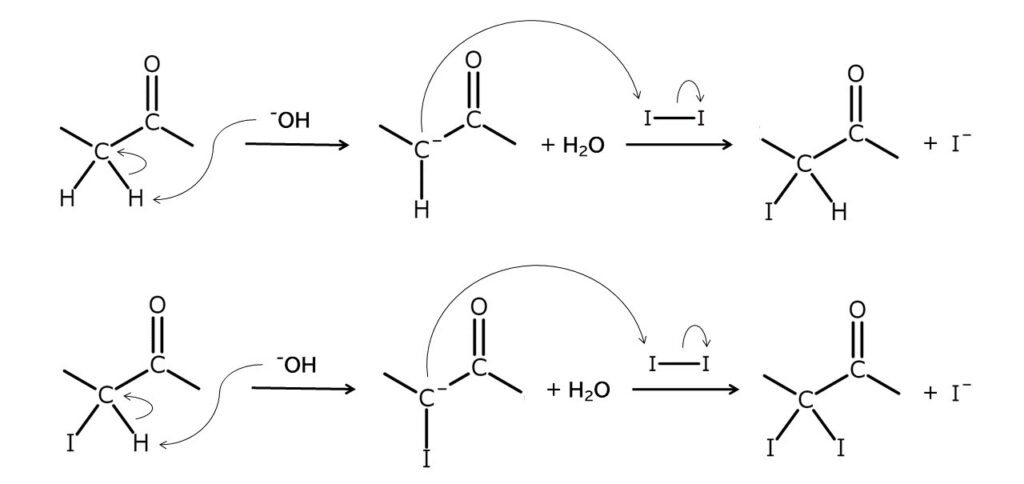
However, there are problems with this method, including low yields, poor consistency, and a tendency to form condensates.
These drawbacks are why the following two methods are often used in the laboratory in place of using elemental iodine.
①Halogen exchange methods
Halogen exchange is used as a method to substitute a halogen atom in a compound with a different halogen atom. In this case, an α-bromoketone or α-chloroketone is reacted with alkali iodide to exchange the bromine atom or chlorine atom in the compound with an iodine atom.
Unfortunately, this method is sensitive to steric hindrances.
To learn more about halogen exchange reactions, see the article below.
②N-Iodosuccinimide (NIS) methods
NIS is an N-iodo compound with a halogen odor and is a white to slightly yellow crystalline powder.
Combining NIS with a protonic acid, such as acetic acid, toluenesulfonic acid, or trifluoroacetic acid, will generate a highly reactive acyl hypoiodite. Such is the reason NIS is utilized as a highly reactive iodinating agent for various applications and can also be used for carbonyl compound iodination.
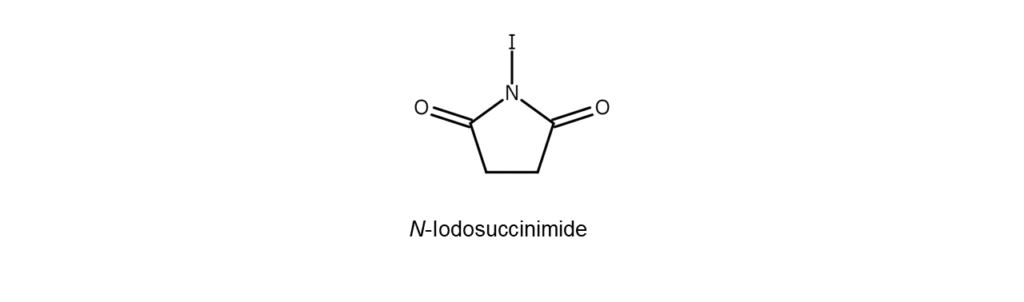
Note, however, that NIS is not well suited for mass synthesis as it is somewhat expensive as a reagent.
Improved methods: Smoother iodination using elemental iodine
As we covered in the section above, traditional iodination methods using elemental iodine have many drawbacks. Regardless, it is still too early to give up on using elemental iodine. There are actually many different improved methods being explored to achieve smoother iodination. Let’s go over the most prominent methods.
Improved method 1:
The first method heats a ketone with elemental iodine and selenium dioxide in acetic acid. This method achieves excellent results with cyclic ketones and ketones with an aromatic ring2.
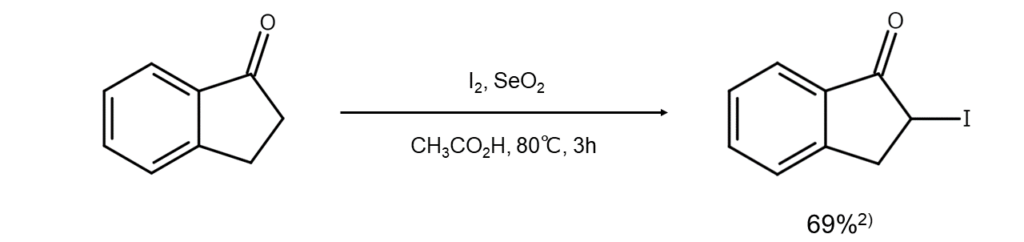
Improved method 2:
This method reacts a ketone with elemental iodine and copper oxide or F-TEDA-BF4 in methanol3,4.
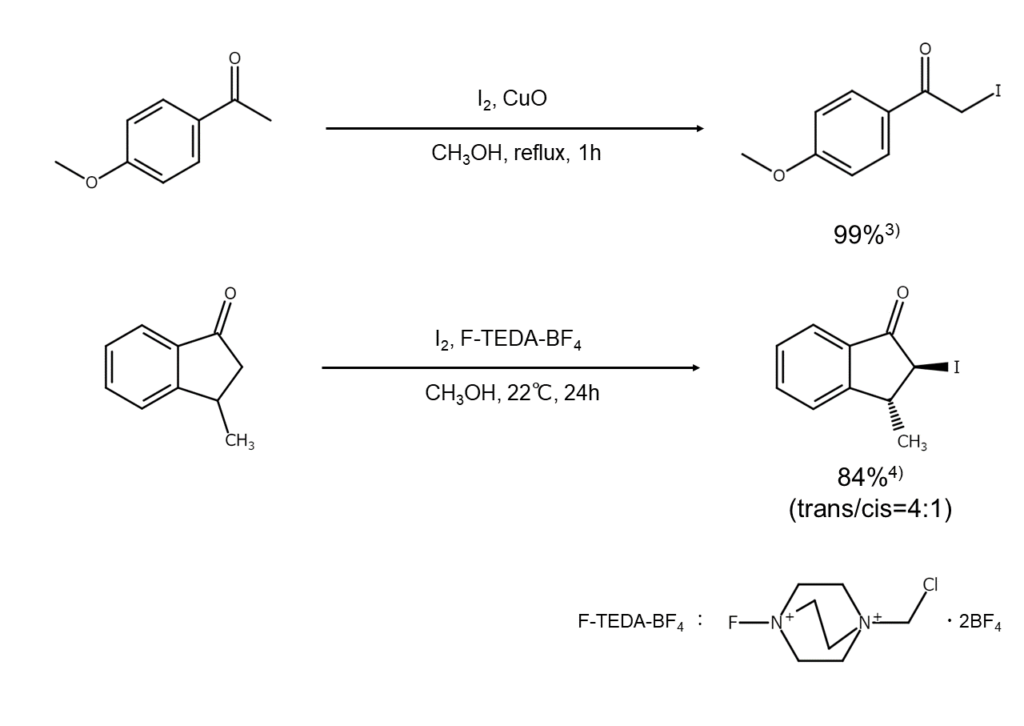
Improved method 3:
In this method, a ketone is first converted into an enol ester with isopropenyl acetate or acetic anhydride and then iodinated with elemental iodine5.
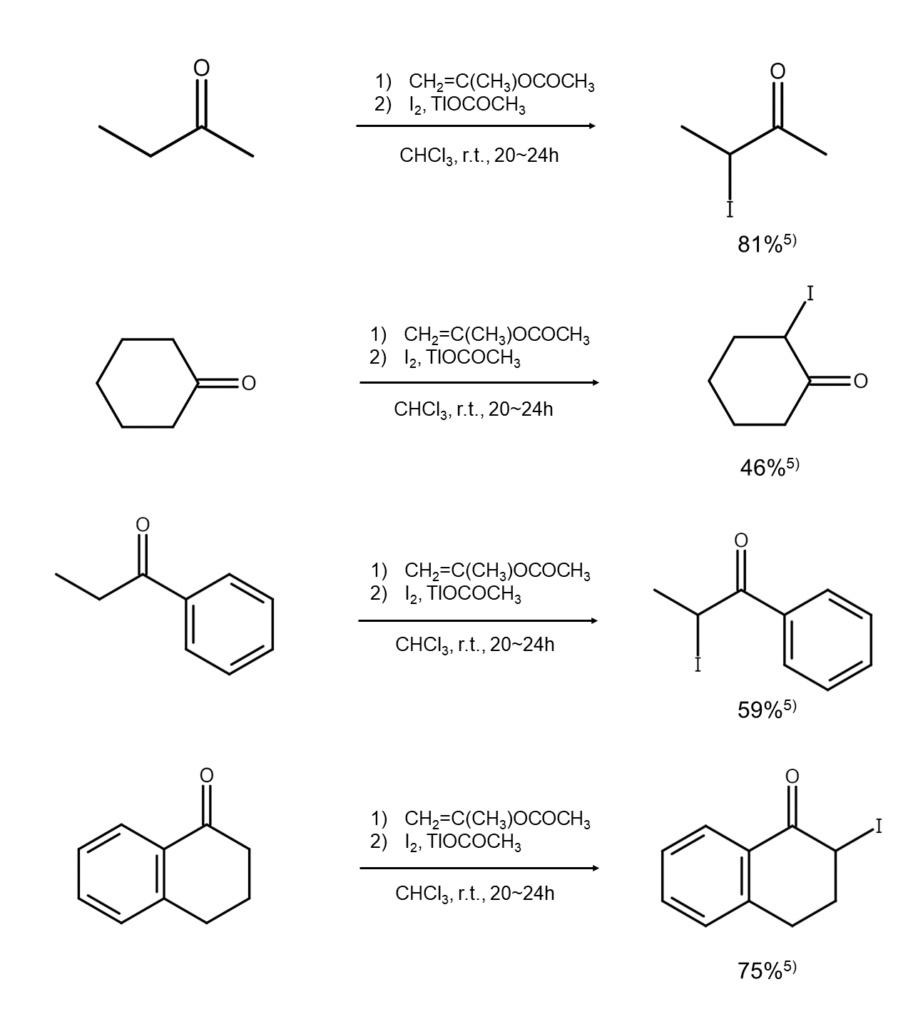
Improved method 4:
This method is proposed for α-iodinating cyclic enones by nucleophilically adding pyridine for conversion into an enolate to then iodinate6.
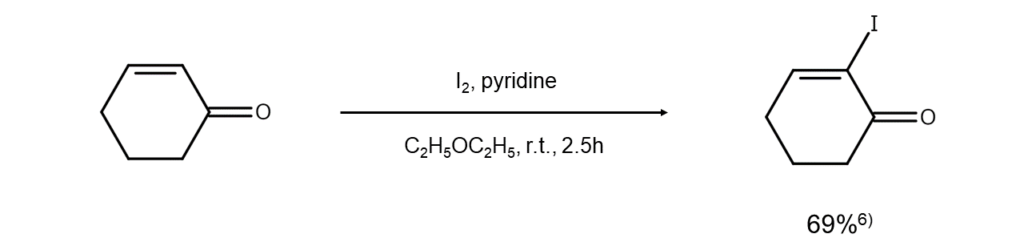
Improved method 5:
α-Iodoketone can be obtained in one step by processing an alkene with iodine and silver chromate under the presence of pyridine. While this reaction provides considerably high yields, it cannot be used with electron-deficient alkenes7.
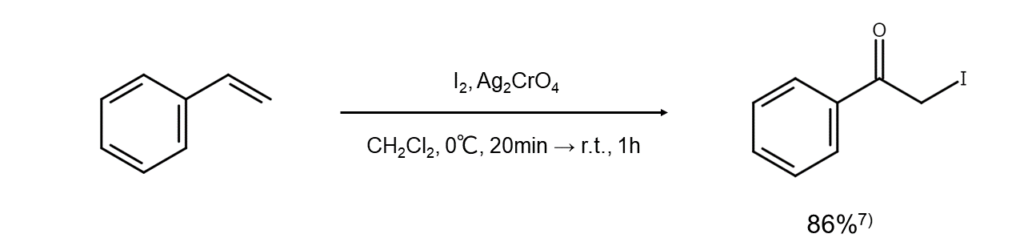
Improved method 6:
Mercury(II) chloride is used together with elemental iodine in this method8. This enables the direct and regiospecific synthesis of α-iodo compounds.
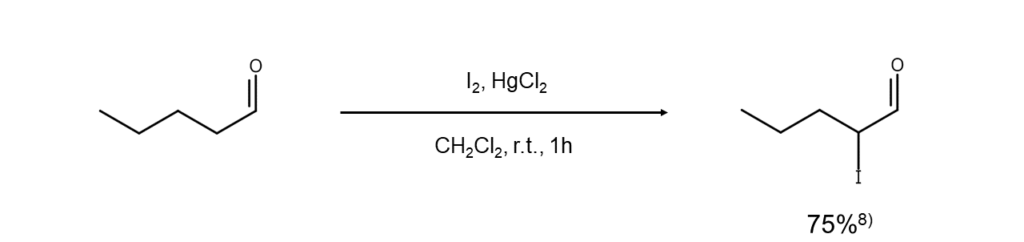
Improved method 7:
This method uses ammonium cerium(IV) nitrate with elemental iodine9. Use this method to obtain high yields of α,α’-diiodoketone.
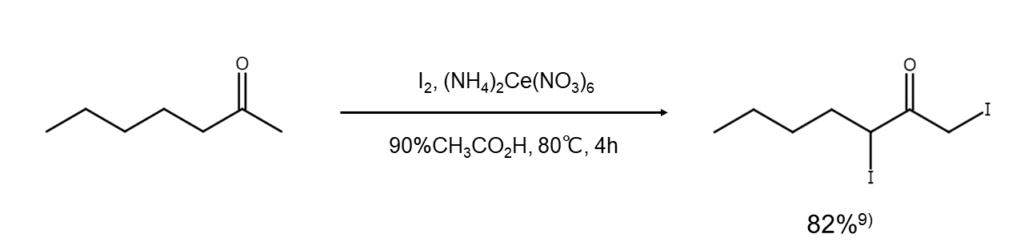
Column: What are the differences between the iodinating agent NIS and the brominating agent NBS?
This article introduced methods that use N-iodosuccinimide (NIS) to conduct carbonyl compound iodination.
There is a compound similar to NIS known as N-bromosuccinimide (NBS). The NBS compound replaces the iodine atom found in the NIS compound with a bromine atom and is well-known as a brominating agent. We have covered bromination reactions that use NBS throughout several articles in this series.

The structures and applications of NIS and NBS may be similar, but the two have several differences.
First, NIS exhibits higher reactivity than NBS. However, unlike NBS, NIS cannot cause allylic iodination even when photo-irradiation is applied or when placed under the presence of peroxide.
There are cost differences between NIS and NBS as well. While NBS is marketed at a relatively low cost, NIS is rather expensive due to the high cost of iodine, its raw material.
Like siblings, NIS and NBS are similar yet different. These two both support our daily lifestyles from behind the scenes.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing. (Japanese)
2) Bekaert A., Barberan, O. et al. Tetrahedron Lett., 2000, 41, 2903.
3) Yin, G., Gao, M. et al. Synthesis, 2007, 3113.
4) Jereb, M., Stavar, S. et al. Tetrahedron, 2003, 59, 5935.
5) Cambie, R. C., Hayward, R. C. et al. J. Chem. Soc. Perkin Trans. 1, 1978, 126.
6) Ruel, F. S., Braun, M. P. et al. Org Synth., 1997, 75, 69.
7) Cardillo, G., Shimizu, M. J. Org. Chem., 1977, 42, 4268.
8) Barluenga, J., Martinez-Gallo, J. M. et al. Synthesis, 1986, 678.
9) Horiuchi, C. A., Takahashi E. Bull. Chem. Soc. Jpn., 1994, 67, 271.















