
Iodination of carboxylic acid and related compounds: Aliphatic compound iodination reactions (5): Discussion series on bromination/iodination reactions 22
Over several articles in this series, we have discussed aliphatic compound iodination reactions using elemental iodine. This article marks the last of this series to discuss aliphatic compounds.
We will close this part of the series with the iodination of carboxylic acid and related compounds (such as ester and acyl halides).
Unlike chlorination or bromination, iodination does not readily occur for carboxylic acids. However, several alternative methods are being proposed as conducive to iodination. Knowledge related to these methods may be useful when designing iodination reactions.
We recommend reading to the end as we discuss details of reaction characteristics and reaction examples throughout the article.
contents
Aliphatic compound iodination reactions with elemental iodine: Iodination of carboxylic acid and related compounds
Several proposed iodination methods
Dissolving iodine in a lower carboxylic acid generates acyl hypoiodite. However, in contrast to the same approach taken with chlorine or bromine, carbon chains will not undergo iodination even if left in this state over an extended period.
In lieu of this approach, a number of iodination methods are being proposed. Let’s go over the details of each one.
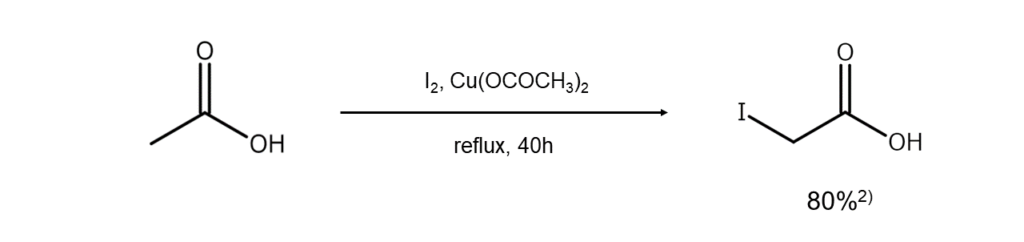
①Copper salt method
Heating a carboxylic acid with iodine in the presence of Cu(OCOCH3)2 or CuCl-CuCl2 (1:1) allows for a high yield of α-iodocarboxylic acid2.
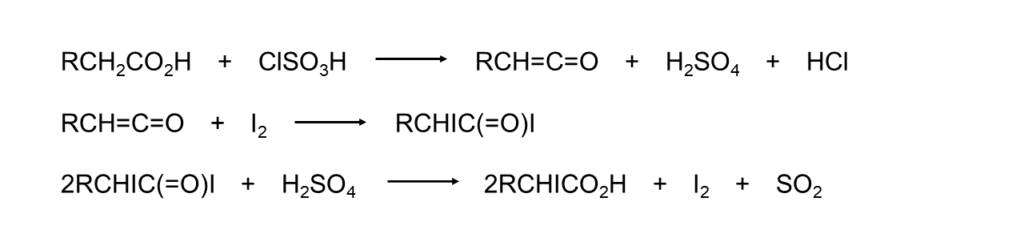
②Chlorosulfonic acid method
Heating iodine and a carboxylic acid in chlorosulfonic acid will yield α-iodocarboxylic acid3.
Since carboxylic acids with a branched structure at the α-position result in low yields, this reaction is believed to take place in two stages, as shown below, the first being the generation of a ketene and the second being the addition of iodine to the ketene.
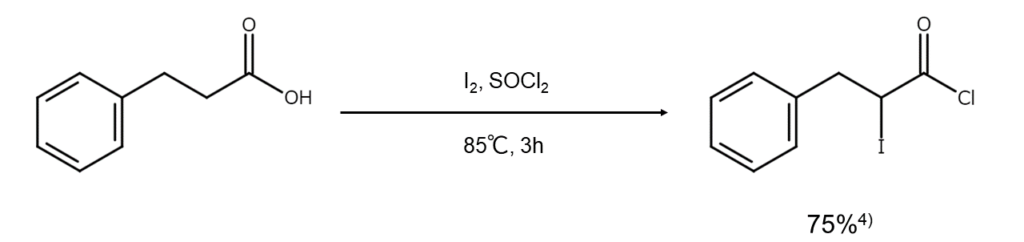
③Thionyl chloride method (acyl chloride iodination)
Heating carboxylic acid in thionyl chloride with iodine produces α-iodoacyl chloride, which is the carboxylic acid derivative known as acyl chloride that has undergone iodination4.
④Lithium dialkylamide method (ester iodination)
This method can be used to obtain an α-iodoester by converting ester, a carboxylic acid derivative, into an ester enolate using lithium dialkylamide and then processing it with iodine5. This is also considered to be an indirect iodination method for carboxylic acids.
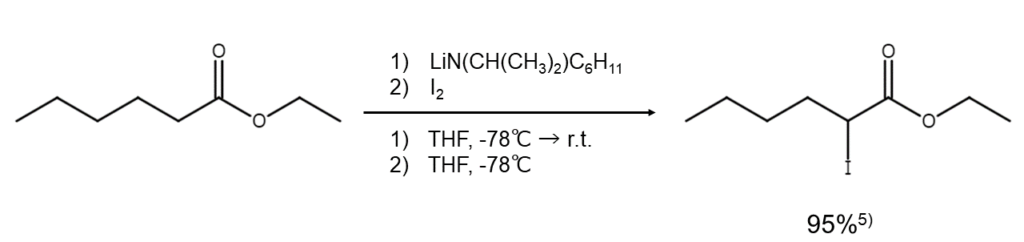
Column: The toxic effects of iodoacetic acid on organisms
Iodoacetic acid, the iodide of acetic acid, is known to be toxic to various organisms. While this compound is better avoided, we may actually be ingesting iodoacetic acid on a daily basis. What exactly does this mean?
In short, it concerns the water we drink every day. Disinfectants such as chlorine are widely used in water processing plants. These disinfectants react with other substances in water, producing compounds known as disinfection byproducts (DBP). Although in low concentration, because DBPs also remain in drinking water, it is believed that we routinely ingest them.
Among these, the DBP that has particularly gained attention for its high cytotoxicity and genotoxicity is iodoacetic acid, which we mentioned above at the beginning of the column.
Many studies have reported on the toxicity of iodoacetic acid. For example, Gonsioroski et al. demonstrated several connections to impacts on reproductive functions, such as changes in the gene expression of mice ovarian cells brought on by iodoacetic acid6.
Water must be disinfected in order to safely drink it, so fully bringing the amount of DBPs generated to zero is not feasible. Nevertheless, new approaches are underway to reduce DBPs by reviewing and revising procedures and processes. We should all take a moment to consider what can be done about DBPs to strike an effective balance.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing. (Japanese)
2) Horiuchi, C. A., Satoh, J. Y. Chem. Lett., 1984, 1509.
3) Ogata, Y., Watanabe, S. J. Org. Chem., 1980, 45, 2831.
4) Harpp, D. N., Bao, L. Q. et al. J. Org. Chem., 1975, 40, 3420.
5) Rathke, M. W., Lindert, A. Tetrahedron Lett., 1971, 3995.
6) Gonsioroski, A., Laws, M. et al. J. Environ. Sci., 2022, 117, 46.










