
Bromination reactions with hydrogen bromide (bromoalkane synthesis from alcohols/bromoalkane synthesis via ether- or lactone-cleaving): Hydrogen bromide (2): Discussion series on bromination/iodination reactions 35
In this series, we discuss bromination and iodination reactions, specialties of MANAC. Last time, we began our exploration of bromination reactions that use hydrogen bromide.
In the previous article, we first explained the characteristics of bromination reactions together with reaction examples. We then discussed the properties and precautions of the hydrogen bromide and hydrobromic acid reagents. The previous article aims to help readers gain an overall understanding of bromination reactions that use hydrogen bromide.
Starting with this article, we move into more detailed discussions of various bromination reactions. First up on the list is bromoalkane synthesis from alcohols and bromoalkane synthesis via ether- or lactone-cleaving. Several handy reaction tips are given throughout the article. Be sure to use them for reference during experiments.
contents
Bromination reactions with hydrogen bromide: Bromoalkane synthesis from alcohols
Reaction mechanisms
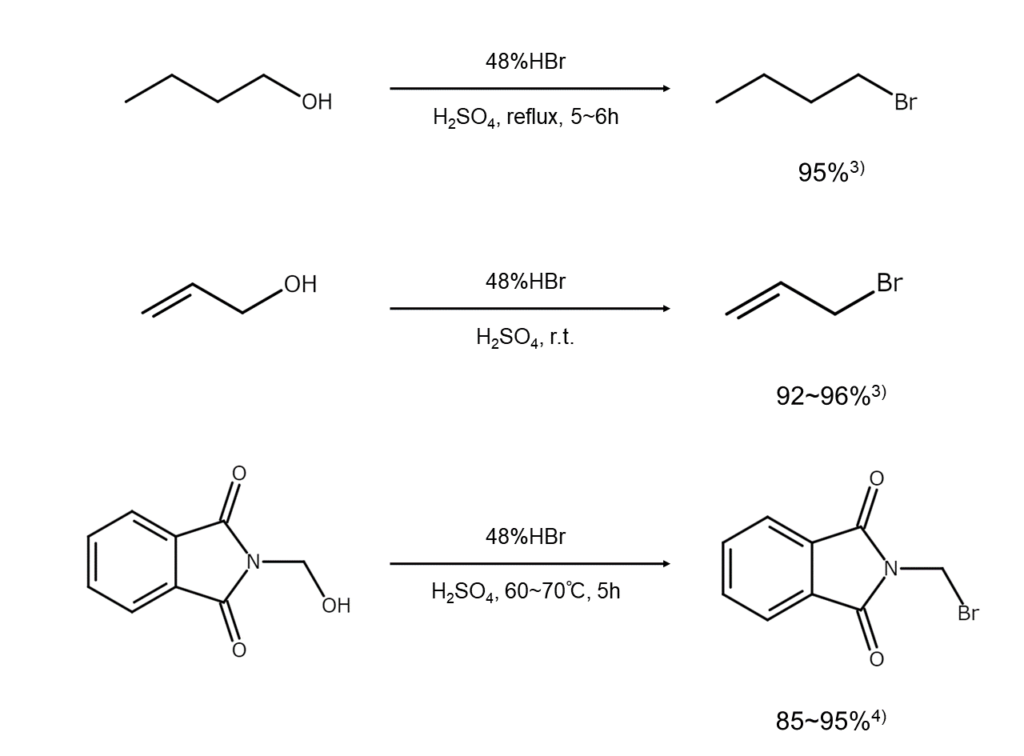
Reacting an alcohol with hydrogen bromide or hydrobromic acid is a standard method for conveniently synthesizing bromoalkanes.
When using a primary alcohol, oxonium ions produced as a result of alcohol protonation are nucleophilically attacked by bromide ions, propelling the reaction forward (Formula 1 below). In the case of a tertiary alcohol, the reaction advances via stable carbocations (Formula 2 below). Secondary alcohols can undergo either of the two reaction mechanisms, an outcome largely determined by reaction conditions.
Formula 1: ROH + H+ → [R-O+H2] + Br– → RBr + H2O
Formula 2: [R-O+H2] → R+ + H2O R+ + Br– → RBr
Caution is required when a halogen, nitro, cyano, carboxy, or any other electron-withdrawing group exists near the hydroxy group in the alcohol, as this will likely hinder protonation and result in incomplete bromination.
Changing reaction conditions according to the alcohol series
Primary and secondary alcohols will normally react with hydrobromic acid through gentle heating. However, in the case of tertiary, allyl, and benzyl alcohols, simply forming a mixture with the reagent and stirring at room temperature will separate an oil layer of bromide.
Caution required toward isomerization and elimination reactions
When reactions progress slowly, favorable results can be obtained by adding small amounts of concentrated sulfuric acid or zinc bromide (ZnBr2) as a catalyst or by raising bromide ion concentration levels by adding sodium bromide (NaBr) to the reaction system. However, caution is required in the case of tertiary alcohols as this type of treatment may sometimes contribute toward the occurrence of isomerization or elimination reactions.
For example, when reacting t-butyl alcohol with hydrobromic acid under heating conditions, the 2-bromo-2-methylpropane generated will undergo partial isomerization and form a 1-bromo-2-methylpropane mixture at a ratio of 4:1.2) Good results are reportedly achievable in many cases for such substrates of concern using different methods. One method dissolves the substrate in a stable organic solvent, and then bubbles dried hydrogen bromide gas into the solution at low temperatures. Another method involves two-phase reactions that employ phase-transfer catalysts (PTC).
The examples below illustrate the synthesis of bromoalkanes from alcohols.
Bromination reactions with hydrogen bromide: Synthesis of bromoalkanes through ether- or lactone-cleaving
Reaction mechanisms
Ethers are cleaved through the action of hydrogen bromide or hydrobromic acid, yielding bromoalkanes and alcohols. This reaction occurs through the nucleophilic attack of bromide ions on the oxonium ions generated as a result of the protonation of ether oxygen (Formula 3 below). In contrast, since benzyl ethers, allyl ethers, and tertiary alkyl ethers readily generate stable carbocations, the reaction progresses through a mechanism in which carbocations are captured by bromide ions (Formula 4 below).
Formula 3: ROR’ + H+ → [R-O+H-R’] + Br– → RBr + R’OH
Formula 4: [R-O+H-R’] → R+ + R’OH R+ + Br– → RBr
Reagents such as sulfuric acid, copper(I) bromide (CuBr), and tetra-alkylammonium bromides can be added to promote the above reactions. For solid ethers, better results can be achieved using hydrogen bromide-saturated acetic acid instead of hydrobromic acid.
Reaction products vary by substrate
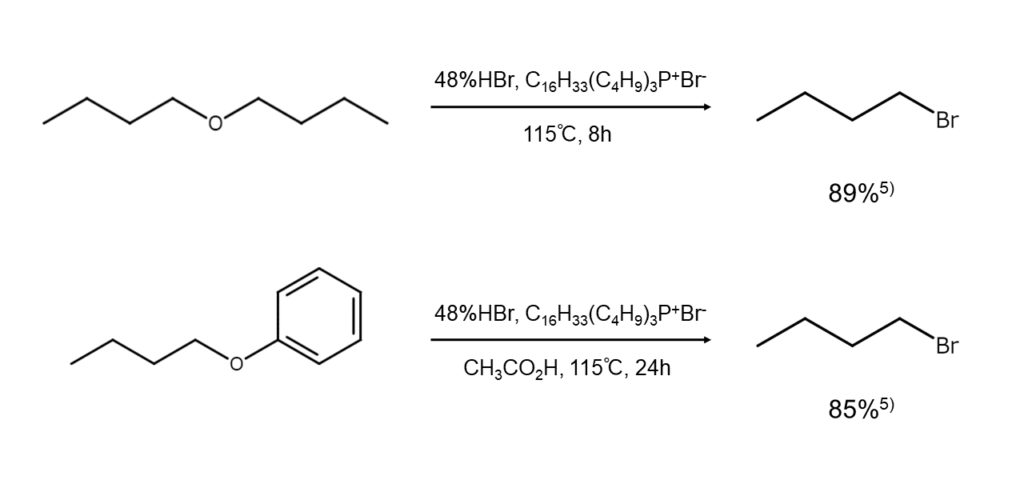
Cleaving unsymmetrical ethers comprised of only primary or secondary alkyl groups yields a bromoalkane mixture with a composition that fluctuates widely depending on the reaction conditions. Furthermore, for methyl ethers (ROCH3) that contain a branched alkyl group (R), methyl bromide (CH3Br) is generated preferentially, while only RBr can be obtained from aromatic ethers (ArOR).
Depending on the substrate, better results may be achieved by using reagents other than hydrogen bromide. For instance, aluminum bromide (Al2Br6) and boron tribromide (BBr3) excel in the brominative cleavage of aromatic ethers. While it is possible to cleave a cyclic ether using acetyl bromide (CH3C(=O)Br), this requires high-temperature heating.
The following are examples of bromoalkane synthesis through ether cleavage.
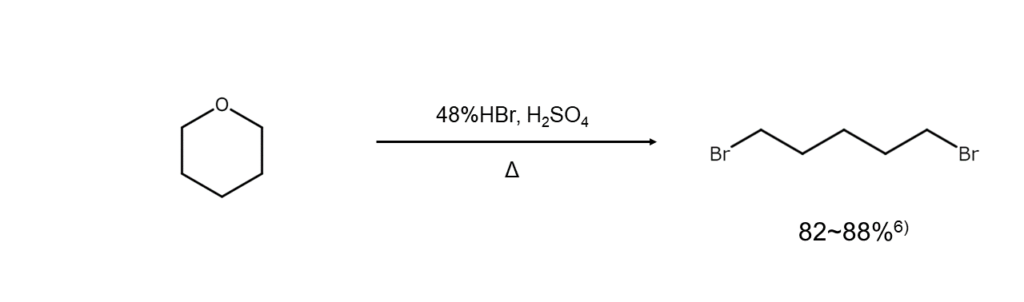
Brominative cleavage of cyclic ethers
When cleaving cyclic ethers with hydrobromic acid, omega-bromoalcohols or alpha, omega-dibromoalkanes can be obtained by adjusting the reagent ratio accordingly. The following is an example of a reaction that can yield alpha, omega-dibromoalkanes.
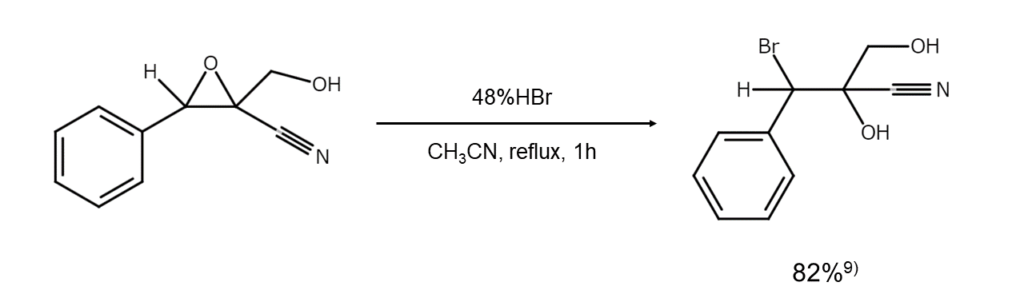
Next, let’s take a look at cleavage reactions of epoxides, a classic example of a cyclic ether. In the brominative cleavage of unsymmetrical epoxides, the carbon atoms with fewer substituents are preferentially brominated. Since the brominative cleavage of epoxycycloalkanes occurs from axial groups, the main reaction product will always be a trans-2-bromo-cycloalkanol.
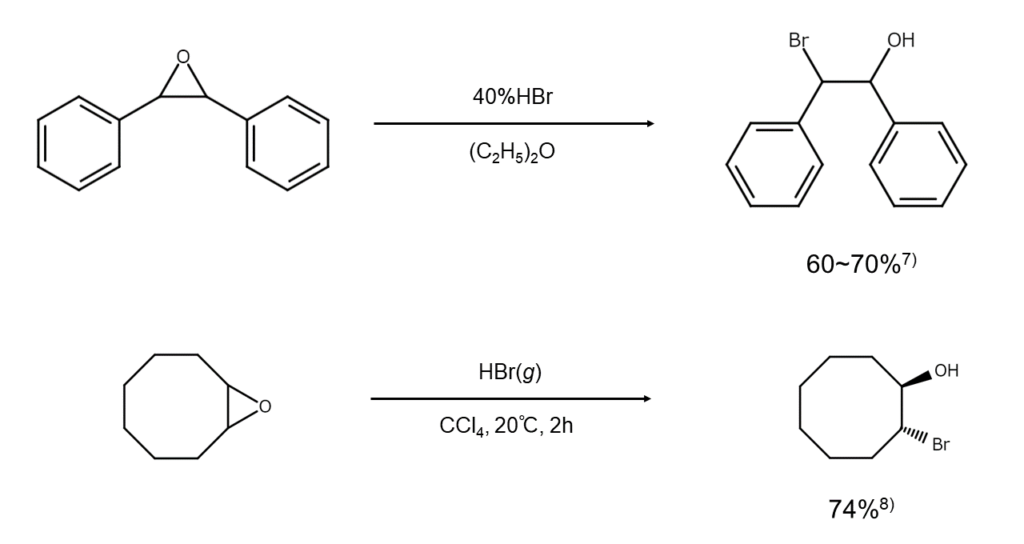
Epoxides are sensitive to acid, so in cases where decomposition or side reactions are a concern, using magnesium bromide (MgBr2) instead of hydrogen bromide is another option.
Column: Aziridine – Brominative cleavage similar to epoxides
We dedicated this article to explaining the brominative cleavage reactions of epoxides, which represent a type of cyclic ether. The explanations aim to help readers understand the reactivity of unsymmetrical epoxides, epoxycycloalkanes, and other epoxides.
Interestingly, there is a compound that exhibits similar reactivity to epoxides during brominative cleavage. That compound is known as aziridine (or ethylene imine).
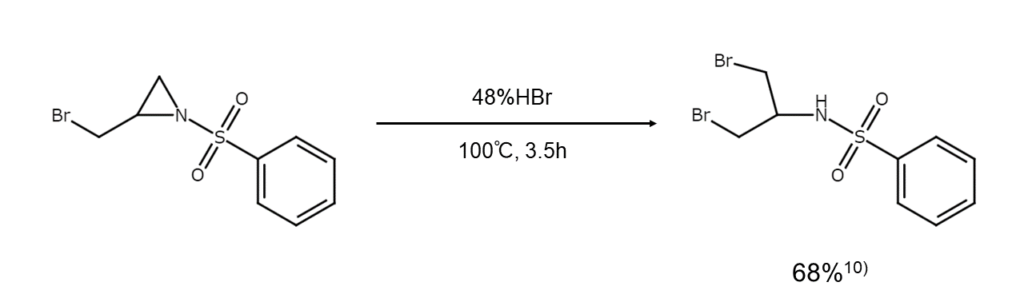
For example, when subjecting asymmetric aziridine to brominative cleavage using hydrogen bromide, the carbon atoms with fewer substituents will be preferentially brominated. This tendency is similar to that of unsymmetrical epoxides.
Brominative cleavage reactions of unsymmetrical epoxides:
Brominative cleavage reactions of asymmetric aziridine:
Epoxide and aziridine – think back on these “two of a kind” compounds whenever you find yourself handling brominative cleavage reactions for cyclic ethers.
MANAC is a global leader in bromination and iodination reactions. Please inquire with us about any bromination or iodination needs.
References
1) MANAC Inc. Research Lab., Suzuki, H. (ed.). Shuuso oyobi Youso Kagoubutsu no Yuuki Gousei: Shiyaku to Gouseihou [Organic Syntheses of Bromine & Iodine Compounds: Reagents & Synthesis Methods], Maruzen Publishing (2017).
2) Michael, A., Sharf, E. et al. J. Am. Chem. Soc., 1916, 38, 653.
3) Kamm, O., Marvel, C. S. Org. Synth. Coll. Vol. 1, 25 (1941).
4) Davidsen, S. K., Phllips, G. W. et al. Org. Synth. Coll. Vol. VIII, 451 (1993).
5) Landini, D., Montanari, F. et al. Synthesis, 1978, 771.
6) Cason, J., Wallcave, W. et al. J. Org. Chem., 1949, 14, 37.
7) Reulos, D. Compt. rend., 1943, 216, 774.
8) Cope, A. C., Johnson, H. E. J. Am. Chem. Soc., 1957, 79, 3889.
9) Layachi, K., Aries-Gautron, I. et al. Tetrahedron, 1992, 48, 1585.
10) Gensler, W. J. J. Am. Chem. Soc., 1948, 70, 1843.













