
Iodonium compounds as initiators: Hypervalent organoiodine compounds (3): Discussion series on bromination/iodination reactions 31
Hypervalent organoiodine compounds have been gaining attention in recent years for potential applications in areas such as green chemistry and energy conservation. Knowledge of hypervalent organoiodine compounds is nothing less than essential for advancing environmentally friendly next-generation research and development.
So far in recent articles, we have discussed dihalogenoiodo compounds and (diacyloxyiodo)arenes, both examples of hypervalent organoiodine compounds. These are useful compounds used as halogenating reagents and oxidants.
In this article, we introduce a certain type of hypervalent organoiodine compound called iodonium compounds. We recommend reading the entire article as we discuss details including structures, properties, and synthesis methods.
contents
Hypervalent organoiodine compounds
Organic compounds that contain hypervalent iodine
A hypervalent organoiodine compound is just that — an organoiodine compound that is hypervalent.
Atoms are “hypervalent” when the most outer electron shell (the part of the atom related to bonds with other atoms), which sits furthest from the nucleus, formally contains more than eight electrons. This state enables the atom to form a higher number of bonds with other atoms. For example, iodine atoms gain the ability to bond with multiple atoms at once when they become hypervalent, as shown in the figures below (the iodine atom on the left is trivalent, while the iodine atom on the right is pentavalent). When an organic compound contains hypervalent iodine, it is known as a “hypervalent organoiodine compound.”
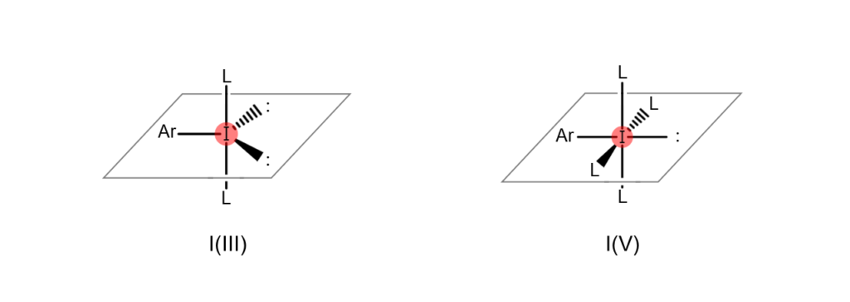
Introducing hypervalent organoiodine compounds: Iodonium compounds
Iodonium compounds
Compounds structured with a divalent iodine cation bonded to two alkyl or aryl groups are called iodonium compounds. The general formula for iodonium compounds is [RR’I+]X–. Among the iodonium compounds, those with OH– in the “X” position of the general formula are called iodonium bases, which are strong bases that exhibit behavior similar to quaternary ammonium bases.
Following IUPAC nomenclature, which classifies hydrogen iodide (HI) as iodane, iodonium compounds are formally classified as λ3-iodanes. However, while typical λ3-iodanes with trivalent hypervalent iodine have a pseudo-trigonal bipyramidal structure,2) the iodonium compounds being introduced in this article differ greatly as they feature a tetrahedral onium structure that satisfies the octet rule.3) Currently, there are no known λ3-iodanes with a tetrahedral structure.
Wide-ranging compounds with varied properties
The stability of an iodonium compound is determined by the structure of the molecules bonded with the iodine atom.
Aliphatic iodonium compounds are not often used in the laboratory as they are less stable than aromatic iodonium compounds. In contrast, aromatic iodonium compounds that are stable enough to undergo long-term storage are not uncommon, and some are even available as reagents.
Aromatic iodonium compounds can be synthesized through any of several different methods, such as the oxidation of an iodoarene under the presence of an arene, the condensation of an iodosyl compound with an arene using an acid catalyst, and the reaction of an organometallic compound with an iodine compound. Examples of aromatic iodonium compound synthesis are shown below.
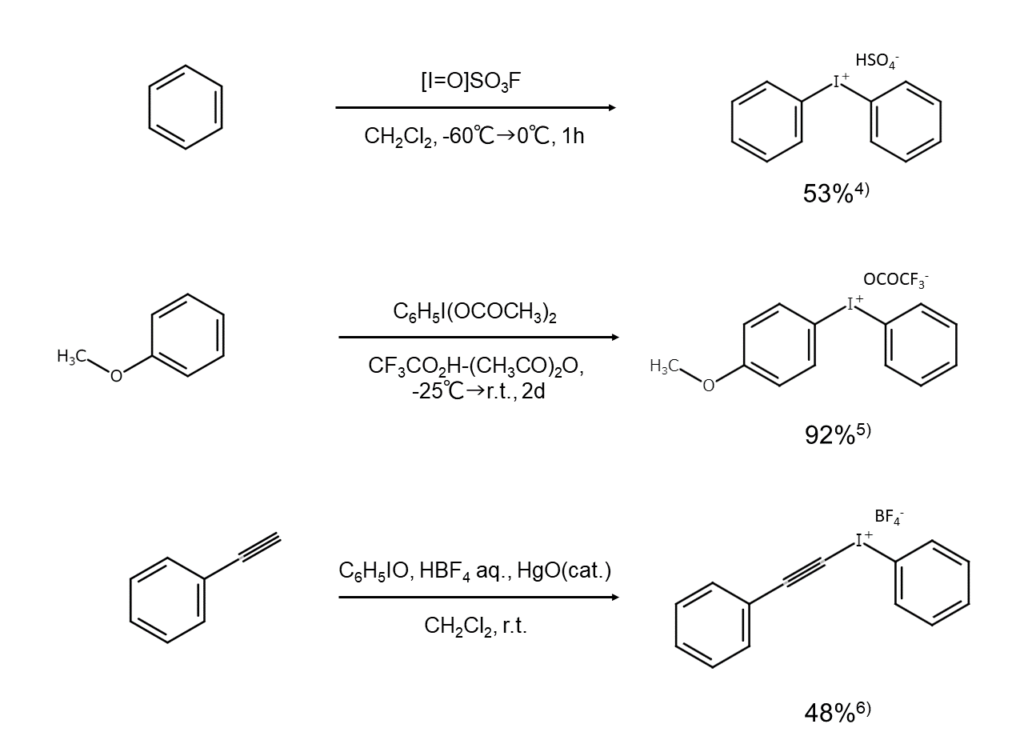
Complex ions capable of isolation as stable crystals
The I+ iodine cation is well established as an active iodinating species, and its existence has been confirmed by spectroscopic and chemical kinetic research. Regardless, extracting this iodine cation in a free state has not been possible.
However, the molecular structures of many compounds have been determined by isolating them as ligand-stabilized complex ions in the form of stable crystals, such as in the case of [I(CH3CN)2]+PF6– and [I(CH3CN)2]+[AgF6]–. Some complex salts are available commercially as reagents.
*To learn more about iodonium compounds, see references 7–10 listed at the end of this article.
Column: Iodonium compounds also useful for dental adhesives?
Did you know that iodonium compounds, the topic of this article, are also important in the field of dentistry?11)12)
Many are familiar with the practice of removing the decayed area of a tooth and replacing the void with a filling when treating a tooth cavity. One type of filling used is a resin material known as composite resin, often referred to as “white filling.” Since composite resin fillings require less of the affected tooth to be removed compared with other filling types, such as metallic and ceramic fillings, they are preferred as a way to preserve more of the natural tooth.
When using a composite resin for dental treatment, a dental adhesive is first applied to the affected tooth area to carry out prepolymerization. The composite resin is then applied over the adhesive, and the site is subjected to curing to harden the applied materials. In this procedure, both the adhesive and the composite resin must be cured. In order to accomplish this, these materials contain components that function as polymerization initiators. A photo radical initiator commonly used is the CQ/amine initiator, which combines camphorquinone (CQ) and a tertiary amine.
There are, however, certain challenges with CQ/amine initiators. While these initiators require exposure to visible light to induce a reaction, the curing rate is slowed by CQ due to its poor absorption of the visible light range. One might wonder, “Why not just add more CQ?” But doing so would increase the likelihood of staining and discoloration. Such implications present limitations to how much CQ concentrations can simply be increased to raise the curing rate in applications for the general public.
It is here that iodonium compounds make their appearance.
Adding an iodonium compound, which is an oxidant, to a CQ/amine initiator is known to accelerate the generation of initiating radicals and increase the curing rate. In fact, research has demonstrated that using a CQ/TMA/DPIH initiator results in up to an approximately fivefold increase in the polymerization rate over using a CQ/TMA initiator (TMA: N,N,3,5-tetramethylaniline; DPIH: diphenyliodonium hexafluorophosphate).13)
While dentistry and iodonium compounds may seem entirely unrelated at first glance, the two are indeed closely intertwined.
MANAC offers manufacturing and marketing services for hypervalent organoiodine compounds. In particular, as we succeeded in significantly lowering our manufacturing costs of (diacetoxyiodo)benzene (DAIB), we can provide high-quality products at a low price.
Please feel free to inquire at the email address below.
chemia@manac-inc.co.jp
References
1) MANAC Inc. Research Lab., Suzuki, H. (ed.). Shuuso oyobi Youso Kagoubutsu no Yuuki Gousei: Shiyaku to Gouseihou [Organic Syntheses of Bromine & Iodine Compounds: Reagents & Synthesis Methods], Maruzen Publishing (2017).
2) Alcock, N. W., Countryman, R. M. J. Chem. Soc., Dalton Trans., 1977, 217.
3) Olah, G. A. et al. Onium Ions, Wiley (1998).
4) Zefirov, N. S., Kasumov, T. M. et al. Synthesis, 1993, 1209.
5) Hossain, Md. D., Ikegami, Y. et al. J. Org. Chem., 2006, 71, 9903.
6) Yoshida, M., Osafune, K. et al. Synthesis, 2007, 1542.
7) Stang, P. J., Zhdankin, V. V. Chem. Rev., 1996, 96, 1123.
8) Zhdankin, V. V., Stang, P. J. Chem. Rev., 2002, 102, 2523.
9) Zhdankin, V. V., Stang, P. J. Chem. Rev., 2008, 108, 5299.
10) Merritt, E. A., Olofsson, B. Angew. Chem. Int. Ed., 2009, 48, 9052.
11) YAMAKIN Co., Ltd. Development Department, “Koubunshi Gijutsu Report vol. 9 Shika Zairyou ni Okeru Kaishizai Seibun Toshiteno Yoodoniumu-en no Riyou”, https://www.yamakin-gold.co.jp/technical_support/webrequest/pdf/kobunshi09_1709w.pdf
12) Sakamoto, T. et al. “Shika Secchakuzai no Kagaku (Chemistry of Dental Adhesives)”, https://www.jstage.jst.go.jp/article/adhesion/52/5/52_5-6/_pdf
13) Cook, W. D., Chen, F. J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 5030.












