Bromination reactions with phosphorus bromides (Phosphorus(V) bromide/phosphoryl bromide): Phosphorus bromides (3): Discussion series on bromination/iodination reactions 41
In this series, we discuss bromination and iodination reactions, specialties of MANAC. This article continues our exploration of bromination reactions with phosphorus bromides.
Here, we take a close look at reactions that use phosphorus(V) bromide and phosphoryl bromide as reagents. Together with our previous coverage of reactions that use phosphorus(III) bromide as a reagent and reactions with bromine and phosphorus, this article should help readers gain an overall understanding of bromination reactions that use phosphorus bromides.
Be sure to read to the end, as the information may also help update your knowledge of bromination reactions.
contents
Bromination reactions with phosphorus bromides: Reactions that use phosphorus(V) bromide as a reagent
Phosphorus(V) bromide basics
Phosphorus(V) bromide (PBr5) is a crystalline solid that fumes in humid air. It exists in two forms: a yellow form and a red form. PBr5 is generally found in the yellow form. When heated, PBr5 will start to sublimate at 83.8°C. Although it does not exhibit a precise melting point, it will begin changing into a red liquid at around 100°C. Since PBr5 is sensitive to moisture, it should either be sealed in an ampoule or stored in a dark environment with inert gas.
Putting PBr5 in water will result in a violent reaction that produces hydrogen bromide (HBr) and yields either phosphoryl bromide (POBr3) or phosphoric acid (H3PO4). However, PBr5 will dissolve without decomposing in certain compounds such as benzene, carbon tetrachloride, dichloroethane, and carbon disulfide. Since PBr5 is toxic and highly corrosive, it must be handled with care as its vapors are harmful to the skin and mucous membranes.
Difficult to use with highly reactive compounds
Depending on the reaction conditions, PBr5 exhibits behavior as an onium salt ([PBr4]+[PBr6]–) or a bromine adduct (PBr3/Br2). However, when its temperature exceeds 100°C, the following dissociation reaction becomes pronounced: PBr5 ⇄PBr3 + Br2. The bromine released during this reaction will readily bring about resinification through oxidation, dehydrogenation, bromination, and other such processes. These mechanisms generally prevent high yields of target bromine compounds, making it challenging to utilize PBr5 with highly reactive compounds.
Alternative compounds to phosphorus(V) bromide
Given the relatively high cost of PBr5, some applications opt for an alternative in the form of a crystalline phosphorus(V) compound reagent obtained by reacting an equimolar amount of bromine with phosphorus trichloride (PCl3) under ice-cold conditions. Although the compound is often given a chemical formula of PCl3Br2, it is not considered a single substance.2), 3)
Reactions that use phosphorus(V) bromide
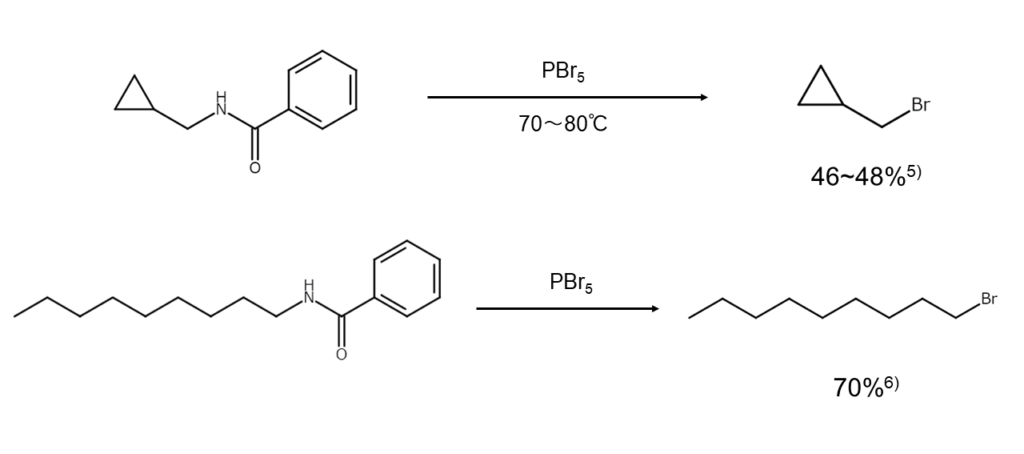
Obtaining bromoalkanes from aliphatic amines through the von Braun reaction
The von Braun reaction is a long-established method famous as a way of obtaining bromoalkanes using PBr5. An RNH2, which is a straight-chain aliphatic amine, is first N-benzoylated. Next, PBr5 is used to convert this into an imidoyl bromide (RN=CBrC6H5) (Formula 1 below). Further subjecting this to thermal decomposition will yield a bromoalkane (RBr) and benzonitrile (C6H5CN) (Formula 2 below).4)
Formula 1: RNHC(=O)C6H5 + PBr5 → RN=CBrC6H5 + POBr3 + HBr
Formula 2: RN=CBrC6H5 → RBr + C6H5CN
The following are examples of common von Braun reactions.
Other reaction examples
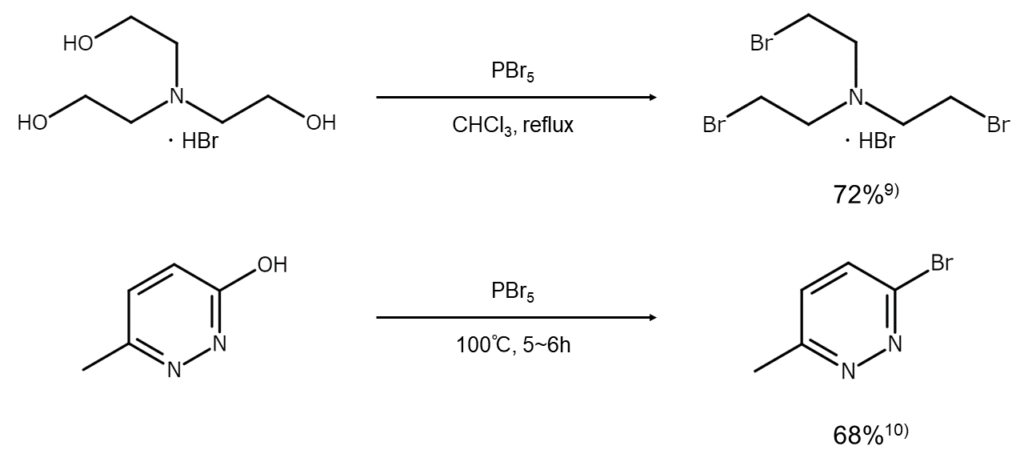
PBr5 is generally used in reactions that substitute the hydroxy groups of an aromatic ring or heteroaromatic ring with bromine atoms, as well as in reactions to convert aldehydes into 1,1-dibromoalkanes. Unlike PBr3, PBr5 is not often used in the conversion of bromoalkanes from alcohols despite having a higher reactivity. However, when converting alcohols into bromides, PBr5 is incredibly effective for alcohols that do not easily react with PBr3, such as those that are structurally hindered and those that have been deactivated by electron-withdrawing groups (alcohols such as polyfluoroalcohol and nitroalcohol).7)
PBr5 is also used when converting a ketone into a gem-dibromide.8) However, care should be taken to ensure it is not approached like phosphorus pentachloride (PCl5), as PBr5 will readily cause alpha-position bromination or aldol condensation.
The following are examples of bromination reactions with PBr5.
Bromination reactions with phosphorus bromides: Reactions that use phosphoryl bromide as a reagent
Phosphoryl bromide basics
Phosphoryl bromide (POBr3) is a colorless to slightly yellow solid with a harsh odor that dissolves in ether, benzene, chloroform, carbon tetrachloride, and hydrogen disulfide. POBr3 easily undergoes hydrolysis with water, which then changes it into hydrobromic acid and phosphoric acid. Contact must be avoided as POBr3 has lachrymatory properties and is also harmful to the skin and mucous membranes.
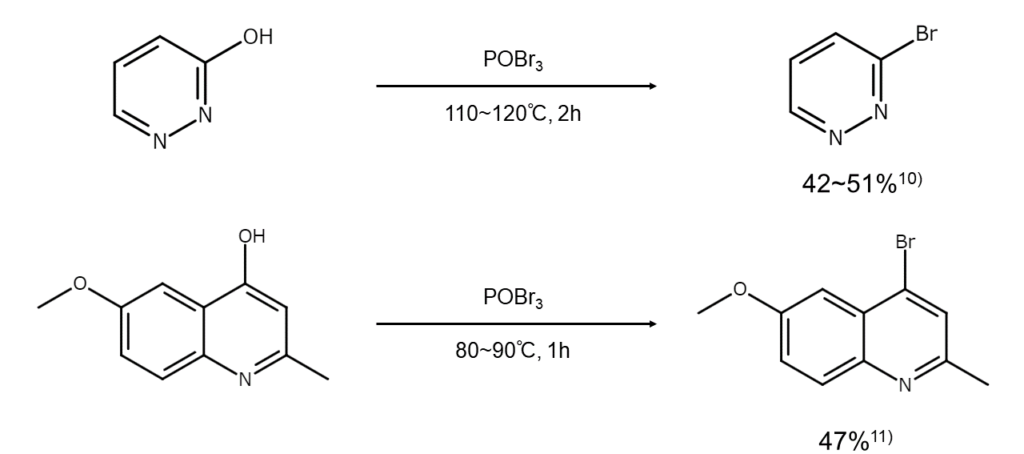
Use in reactions that substitute hydroxy groups with bromine atoms
POBr3 is used when substituting the hydroxy groups of aromatic rings and heterocycles with bromine atoms. Since its bromination capacity is not as high as that of PBr5, POBr3 can be considered a safe reagent. In general, reactions are carried out by dissolving the substrate in toluene or xylene, adding excess POBr3, and heating the mixture to 110°C–160°C until the reaction ceases to generate HBr.
The following are examples of bromine compound synthesis using POBr3.
Column: Appearances can be deceiving – A look at the main differences between the seemingly similar PBr3 and PBr5
While phosphorus(III) bromide (PBr3), the focus of a previous article, and phosphorus(V) bromide (PBr5), our topic in this article, are both comprised of phosphorus and bromine, there are many differences between the two compounds. The major differences are shown in the table below.
| PBr3 | PBr5 | |
| Properties under normal temperature/pressure | Colorless liquid | Red or yellow crystalline solid |
| Structure | Trigonal pyramidal molecule | Ionic crystal with a [PBr4]+Br– structure (solid state)* *Note: [PBr4]+ has a tetrahedral structure. |
| Stability | More stable than PBr5 | Less stable than PBr3* *Note: Decomposes into PBr3 and Br2 when over 100°C. |
The differences in structure are most striking. In contrast to PBr3 being a trigonal pyramidal molecule, PBr5 exists as an ionic crystal with a [PBr4]+Br– structure when in a solid state.
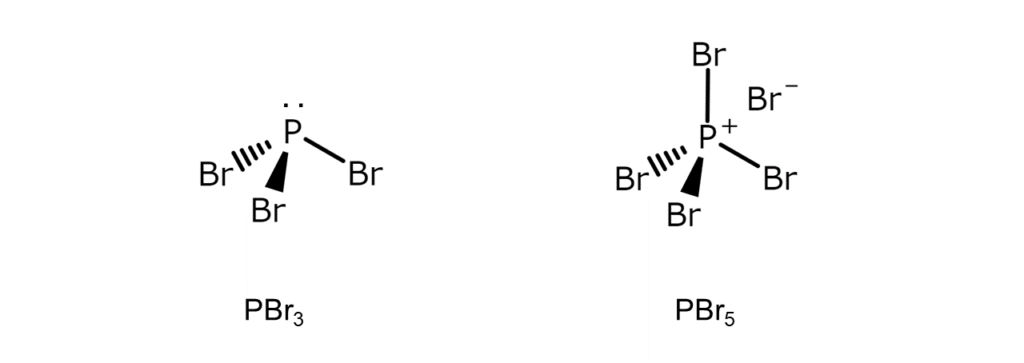
Contrary to their similar chemical formulas, PBr3 and PBr5 sport drastically different structures under normal temperatures and pressures. You might say that these two substances truly embody the mystique of chemistry.
References
1) MANAC Inc. Research Lab., Suzuki, H. (ed.). Shuuso oyobi Youso Kagoubutsu no Yuuki Gousei: Shiyaku to Gouseihou [Organic Syntheses of Bromine & Iodine Compounds: Reagents & Synthesis Methods], Maruzen Publishing (2017).
2) Popov, A. I., Geske, D. H. et al. J. Am. Chem. Soc., 1956, 78, 1793.
3) Harris, G. S., Payne, D. S. J. Chem. Soc., 1956, 4613.
4) von Braun, J. Org. Synth. Coll. Vol. I, 428 (1941).
5) Arvin, J. A., Adams, R. J. Am. Chem. Soc., 1928, 50, 1983.
6) von Braun, J., Irmisch, G. Chem. Ber., 1932, 65, 880.
7) Eliel, E. L., Haber, R. G. J. Org. Chem., 1959, 24, 143.
8) Kutyrev. A. A., Biryukov, V. V. et al. Tetrahedron, 1990, 46, 4333.
9) Childs, A. F., Goldsworthy, L. J. et al. J. Chem. Soc., 1948, 2174.
10) Grundmann, C. Chem. Ber., 1948, 81, 1.
11) Slater, R. H. J. Chem. Soc., 1931, 107.