
Benzylic brominations/aromatic ring brominations: DBDMH bromination reactions: N-bromo compounds (9): Discussion series on bromination/iodination reactions 9
In this series, we discuss bromination and iodination reactions, specialties of MANAC. This article introduces DBDMH, an intriguing brominating agent capable of overcoming the limitations of NBS.
DBDMH is characterized by reduced bromination costs and byproduct amounts over NBS, the commonly used brominating agent, while maintaining a similar reactivity. DBDMH is not as widely known as NBS but has a strong following as a brominating agent in various fields.
Many of you who have read this far likely have used NBS routinely but are now interested in giving DBDMH a try. Surely, many of you would like to learn more about the characteristics of DBDMH.
In this article, we review two types of reactions that utilize DBDMH: (1) reactions that brominate the benzylic position, and (2) reactions that brominate aromatic rings in a stepwise process. This article details reaction mechanisms and example reactions, and is geared toward providing readers with useful information for research and experiments.
■ What you can learn from this article ✔ By using DBDMH, it is possible to selectively monobrominate methyl or methylene groups adjacent to aromatic rings. ✔ By coexisting trimethylsilyl triflate, the reactivity of DBDMH is enhanced, allowing for easy bromination of compounds such as benzoic acid esters that typically do not react with DBDMH. ✔ The type of catalyst determines the bromination reaction, with Lewis acids favoring benzylic bromination and Brønsted acids promoting bromination of the aromatic ring. ■ Recommended Articles ・ A brominating agent that facilitates addition reactions with alkenes, overview and reaction mechanisms of N-bromoacetamide (NBA): N-bromo compounds (10): Discussion series on bromination/iodination reactions 10 ・ A fascinating brominating agent capable of minimizing costs and byproducts, describing 1,3-dibromo-5,5-dimethylhydantoin (DBDMH): N-bromo compounds (8): Discussion series on bromination/iodination reactions 8
contents
Describing 1,3-dibromo-5,5-dimethylhydantoin (DBDMH)
NA brominating agent with similar reactivity to NBS, composed of molecules containing two bromine atoms
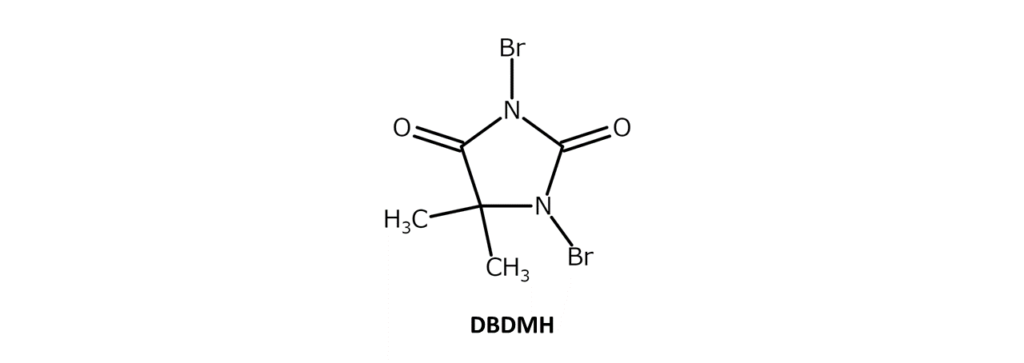
DBDMH is a slightly yellow to yellow-red crystalline powder with a distinct, weak odor and a melting point of 197–199°C (decomposition). The compound is soluble in substances such as ethanol and chloroform, slightly soluble in acetone, dioxane, THF, hot water, and boiling carbon tetrachloride, and has poor solubility in hexane. DBDMH is also called dibromantin.
Since DBDMH powder is easier to handle than bromine (liquid), it is often used as a brominating agent in organic syntheses. With its ability to undergo long-term storage in cool, dark, dry places, DBDMH is also relatively inexpensive. While DBDMH exhibits nearly identical reactivity to NBS, the two bromine atoms in the DBDMH compound enable major advantages over NBS with lower bromination costs and reduced amounts of imide byproducts.
Please see this article for information on the characteristics and precautions of DBDMH and an overview of DBDMH bromination reactions.
DBDMH bromination reactions: bromination of the benzylic position
Reaction details
Using DBDMH allows for the selective monobromination of methyl groups or methylene groups adjacent to aromatic rings. This reaction is of the Wohl-Ziegler type, similar to NBS allylic and benzylic brominations, and progresses as a radical chain reaction.
The expected reaction mechanism entails a series of steps. First, when the N-Br bond present in DBDMH is radically cleaved, the resulting bromine radicals extract the benzylic hydrogen atoms, forming benzyl radicals and HBr. This HBr then reacts with the bromine atoms of the DBDMH, forming Br2 in situ. The Br2 reacts with the benzyl radicals mentioned above, brominating the benzylic position. Additional bromine radicals are generated in the bromination process, resulting in a radical chain reaction.
The bromination reaction described above is known to typically occur in the following order: CH2 (secondary carbon) → CH3 (primary carbon) → CH (tertiary carbon). When bromination reactions progress slowly, there are cases where adding a radical generator (5–10%) or applying photo-irradiation is effective.
The figure below illustrates an example of a benzylic bromination reaction using DBDMH.
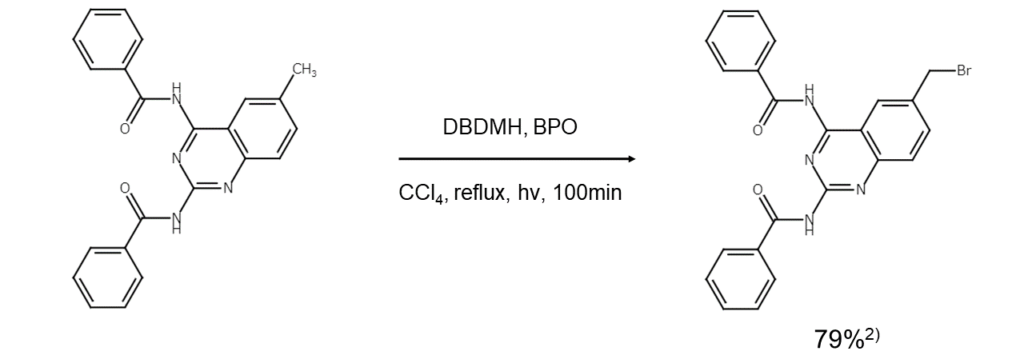
DBDMH bromination reactions: bromination of the benzylic position
Reaction details
The nitrogen atom in DBDMH is adjacent to an electron-withdrawing carbonyl group, making the bromine atoms in DBDMH positively polarized and enabling them to act electrophilically toward electron-rich compounds. Consequently, using DBDMH allows for a stepwise bromination of aromatic rings activated by electron-donating groups under the presence of an acid catalyst (aromatic electrophilic substitution reaction).
Furthermore, the reactivity of DBDMH increases when combined with trimethylsilyl triflate (TMSOTf), which allows for readily brominating compounds that normally do not react with DBDMH, such as benzoic acid esters. In their article published in the organic chemistry journal Tetrahedron Letters, Chassaing and others describe that the increased reactivity seen in the “DBDMH-TMSOTf mixture…could be explained by the formation of a bromonium triflate as a reactive intermediate.”3
Examples of aromatic ring bromination reactions using DBDMH are shown in the figures below.
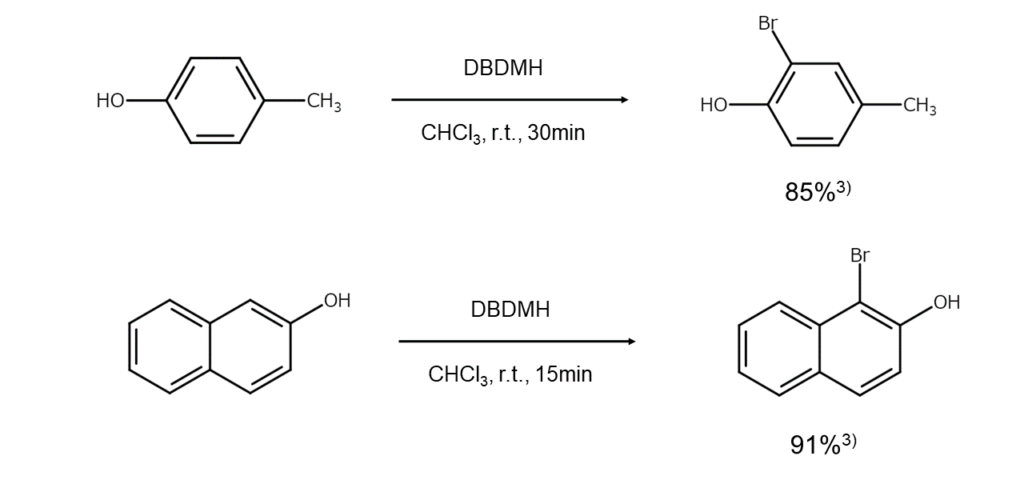
Bromination positions determined by the catalyst used
There is research that demonstrates that the catalyst used in a bromination reaction determines whether benzylic bromination or aromatic ring bromination will occur preferentially.5
The research reports that benzylic bromination readily occurs when Lewis acid catalysts such as zirconium(IV) chloride are used, and that aromatic ring bromination readily occurs with Brønsted acid catalysts such as trifluoromethanesulfonic acid. Lewis acids facilitate the generation of benzyl radicals and promote Wohl-Ziegler benzylic bromination reactions. Likewise, because Brønsted acid catalysts protonate DBDMH, they promote Friedel-Crafts electrophilic aromatic ring bromination reactions, resulting in this difference in bromination position.

MANAC manufactures and sells NBS, the commonly known N-bromo compound, as well as DBDMH. Please feel free to inquire.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing.
2) Oakes, V., Rydon, H. N. et al. J. Chem. Soc., 1962, 4678.
3) Chassaing, C., Haudrechy, A. et al. Tetrahedron Lett. 1997, 38, 4415.
4) Alam, A., Takaguchi, Y. et al. J. Fac. Environ. Sci and Tech., Okayama Univ., 2005, 10, 105.
5) Shibatomi, K., Zhang, Y. et al. Chem Asian J. 2008, 3, 1581.














