Allylic position and benzylic position bromination: Bromination reactions that use NBS (1): N-bromo compounds (3): Discussion series on bromination/iodination reactions 3
In this series, we discuss bromination and iodination reactions, specialties of MANAC. Starting with this issue, we will review various bromination reactions that use NBS.
This article discusses bromination of the allylic position and benzylic position, which can be described as representative of reactions that use NBS. Basic principles behind the reactions, precautions, and examples of reactions are presented for use as a helpful reference.
■ What you can learn from this article ✔ Representative reactions of NBS include bromination at allylic and benzylic positions. ✔ In some cases, combining bromination and debromination reactions allows for selective synthesis of monobrominated compounds. ✔ MANAC manufactures and sells NBS and DBDMH, two prominent N-bromo compounds. ■ Recommended Articles ・Active/inactive aromatic ring bromination: Bromination reactions that use NBS (2): N-bromo compounds (4): Discussion series on bromination/iodination reactions 4 ・Benzylic brominations/aromatic ring brominations: DBDMH bromination reactions: N-bromo compounds (9): Discussion series on bromination/iodination reactions 9 ・The alpha-bromo compound series – Determining acidic or alkaline qualities
contents
Describing N-bromosuccinimide (NBS)
A very commonly used, easy-to-handle brominating agent
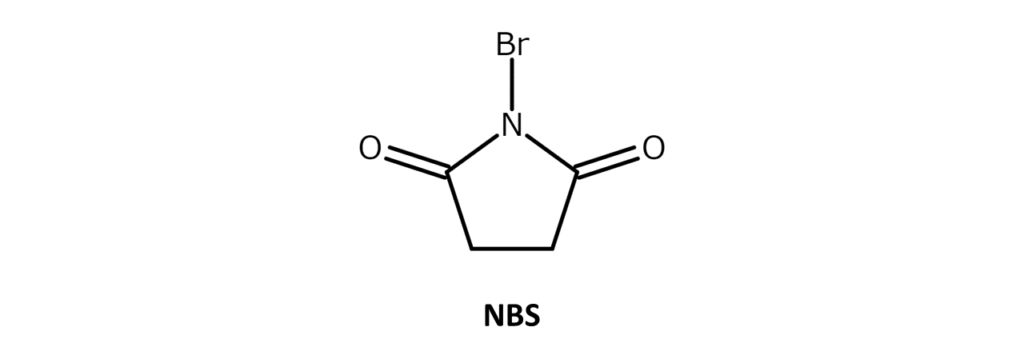
NBS is a white to slightly yellow crystalline powder with a weak bromine odor and a melting point of 173–176°C (decomposition). The compound is soluble in substances such as acetone, THF, DMF, acetonitrile, and DMSO, slightly soluble in water and acetic acid, and has poor solubility in hexane and carbon tetrachloride.
NBS powder is easier to handle than bromine (liquid), making it a common first-line brominating agent in organic syntheses. With its ability to undergo long-term storage in cool, dark, dry places, NBS is also relatively inexpensive.
This article covers information regarding the characteristics and precautions of NBS and an overview of bromination reactions that use NBS.
Bromination reactions that use NBS: Allylic position and benzylic position bromination
Reaction details
Bromination of the allylic position and benzylic position is representative of NBS reactions. This is also known as the Wohl-Ziegler reaction, which progresses into a radical chain reaction.
The reaction mechanism shown below is an example of allylic position bromination. First, when the N-Br bond present in NBS is radically cleaved, the resulting bromine radicals extract the hydrogen atoms bonded to the bromine at the allylic position, forming allyl radicals and HBr. This HBr then reacts with different NBS, forming Br2 in situ. The Br2 reacts with the allyl radicals mentioned just earlier, brominating the allylic position. Additional bromine radicals are generated in the bromination process, resulting in a radical chain reaction.
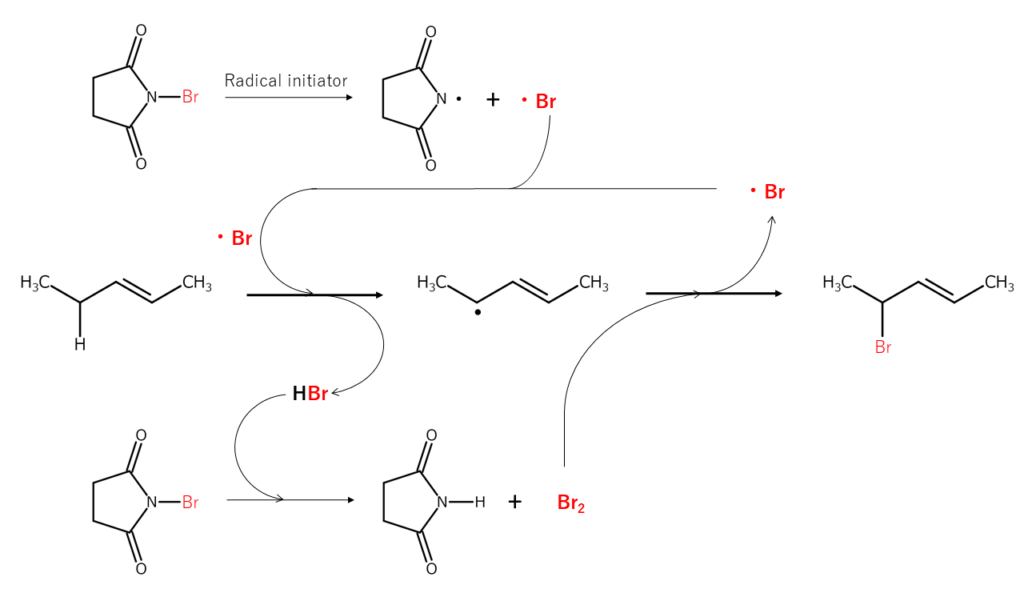
The bromination reactions shown above are known to typically occur in the following order: CH2 (secondary carbon) > CH3 (primary carbon) > CH (tertiary carbon). When bromination reactions progress slowly, there are cases in which adding a radical generator (5–10%) or applying photo-irradiation is effective. Solvents used for these reactions include carbon tetrachloride, benzene, and cyclohexane. Since solvents also affect bromination selectivity2, it is important to consider the effects of solvents when planning a reaction.
N-bromophthalimide, another N-bromo compound, can also be used for allylic position bromination. However, for reasons including its low reactivity, there are few advantages in using N-bromophthalimide over NBS for synthesis.
The figures below illustrate examples of allylic position and benzylic position bromination reactions using NBS.
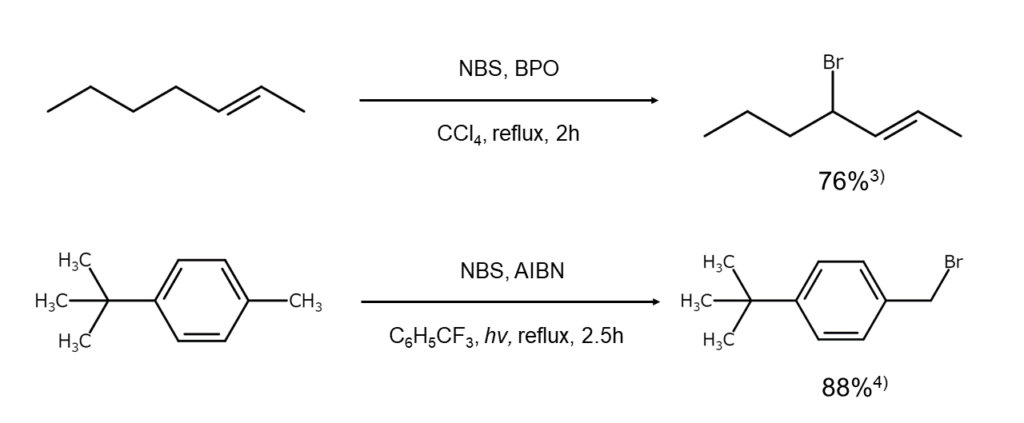
Selectively obtaining monobrominated compounds
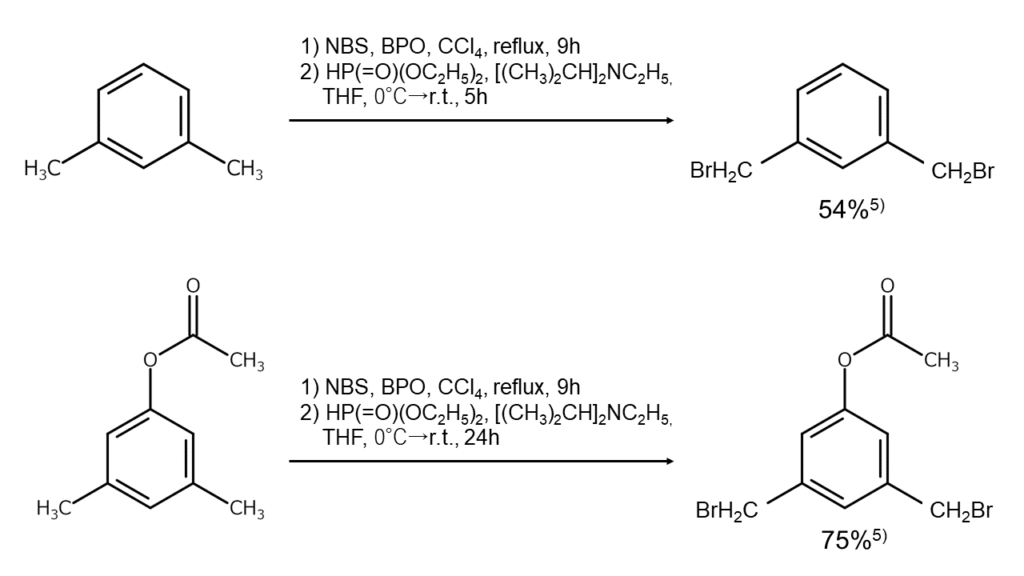
In certain cases, monobrominated compounds can also be selectively obtained by combining bromination reactions with debromination reactions. As an example, if debromination is conducted reductively with diethyl phosphite and N,N-diisopropylethylamine after the benzylic position has been polybrominated using excess NBS under the presence of a radical initiator, compounds monobrominated at the benzylic position, such as that shown below, can be selectively obtained alone5.
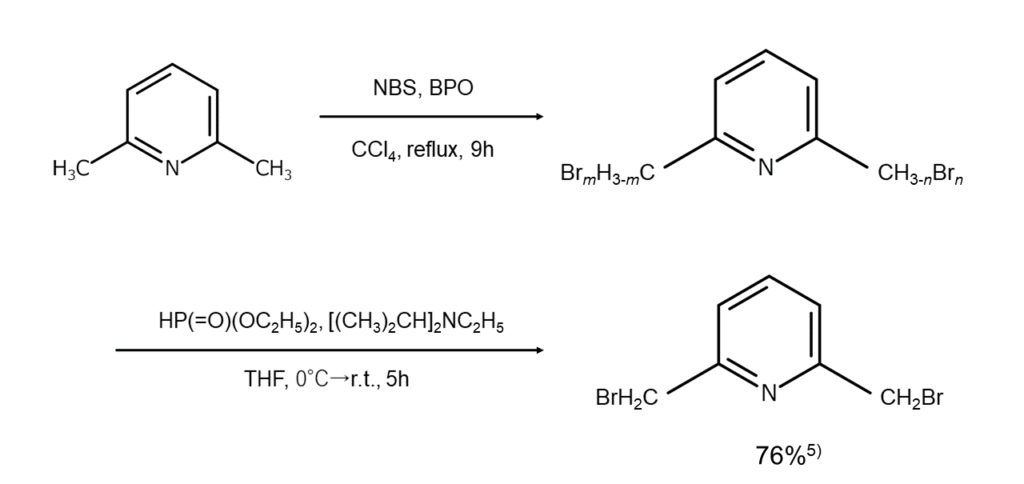
The figures below illustrate examples of reactions where the benzylic position is selectively monobrominated.
[MANAC Tidbits] Handle allyl bromide and benzyl bromide with care
Allyl bromide and benzyl bromide, formed through bromination reactions at the allylic and benzylic positions, are highly reactive bromine compounds often used as chemical intermediates. However, depending on the person, these compounds may lead to symptoms such as rashes if adhered to skin or if vapors are inhaled. Furthermore, caution is required for highly volatile compounds as handling even small amounts in a laboratory may result in eye pain and skin irritation.
Precautions, including the use of fume hoods and wearing protective equipment, are crucial not only when synthesizing allyl bromide or benzyl bromide but also when these compounds may form as byproducts.
MANAC manufactures and sells NBS and DBDMH, two common N-bromo compounds. Please feel free to inquire.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing
2) Offermann, W,. Vogtle, F. Angew. Chem. Internat. Ed., 1980, 92, 471.
3) Greenwood, F. L., Kellert, M. D. J. Am. Chem. Soc., 1953, 75, 4842.
4) Suarez, D., Laval, G. et al. Synthesis, 2009, 1807.
5) Liu, P., Chen, Y. et al. Synthesis, 2001, 2078.