One of the most powerful brominating agents, overview and reaction mechanisms of dibromoisocyanuric acid (DBI): N-bromo compounds (11): Discussion series on bromination/iodination reactions 11
This issue marks the last of this series to discuss bromination reactions using N-bromo compounds.
Leading up to this issue, we have delved into many brominating agents, including N-bromosuccinimide (NBS), a first-line brominating agent in the laboratory; 1,3-dibromo-5,5-dimethylhydantoin (DBDMH), capable of reducing brominating costs and byproduct amounts; and N-bromoacetamide (NBA), with its ability to prioritize addition reactions. Each of these brominating agents has its own special characteristics.
In this final issue, we review another brominating agent just as unique and valuable. That agent is dibromoisocyanuric acid (DBI), which boasts a brominating ability that ranks top-class among N-bromo compounds. Compounds not readily brominated by other N-bromo compounds can be brominated with relative ease when using DBI.
In this article, we discuss DBI’s characteristics, handling precautions, and bromination reactions, and close with a brief overview of some N-bromo compounds not covered in this series.
■ What you can learn from this article ✔ DBI is a mild yet powerful brominating agent that exhibits stronger brominating ability than NBS. ✔ By using DBI, it is possible to achieve bromination of normally challenging inactive aromatic rings under mild conditions. ✔ Introducing N-bromo compounds other than NBS, DBDMH, NBA, and DBI that exist in the world. ■ Recommended Articles ・ A brominating agent that facilitates addition reactions with alkenes, overview and reaction mechanisms of N-bromoacetamide (NBA): N-bromo compounds (10): Discussion series on bromination/iodination reactions 10
contents
Describing dibromoisocyanuric acid (DBI)
A powerful brominating agent capable of gently brominating inactive aromatic rings
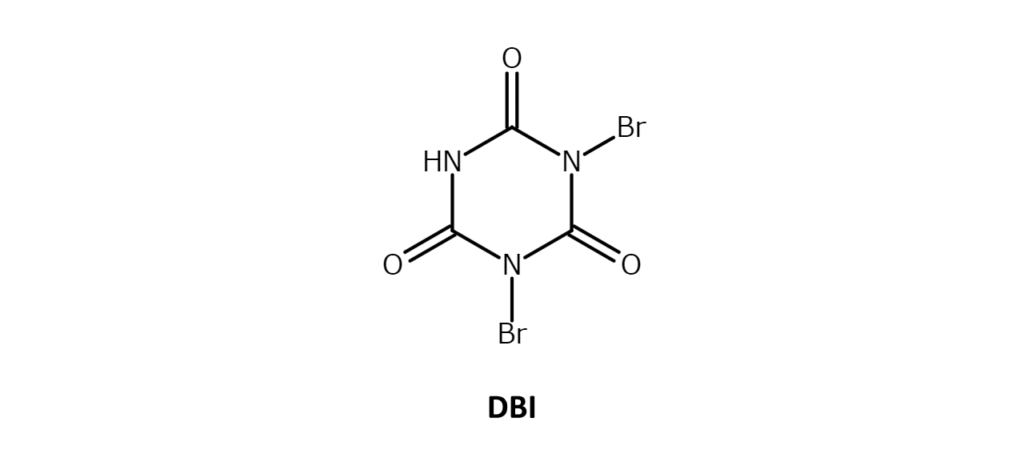
Dibromoisocyanuric acid (DBI) is a white to slightly yellow crystalline powder with a melting point of 309°C. Although soluble in dichloromethane and concentrated sulfuric acid, the DBI compound exhibits poor solubility in common organic solvents.
DBI is a gentle yet powerful brominating agent capable of brominating inactive aromatic rings under mild conditions. Reaction details are covered below.
DBI preparation method and precautions
DBI can easily be prepared through a method in which bromine is set to act on isocyanuric acid in an alkali hydroxide aqueous solution.2 However, because factors such as moisture and light degrade DBI, it must be kept in cool, dark, dry storage.
DBI handling precautions
There is a risk of DBI igniting or exploding if it comes into contact with reducing agents or is mixed with combustibles. As DBI is a severe irritant for skin and mucous membranes, adequate care must be taken to prevent such contact.
Bromination reactions using DBI: inactive aromatic ring bromination
Reaction details
Bromine atoms in N-bromo compounds are positively polarized and act electrophilically on compounds rich in electrons, making it relatively simple to use N-bromo compounds to brominate aromatic rings activated by electron-donating groups. Conversely, aromatic rings deactivated by electron-withdrawing groups are challenging to brominate.
It is here that DBI makes its appearance. Even under mild reaction conditions, DBI makes the normally unobtainable inactive aromatic ring bromination obtainable, made possible by none other than the powerful brominating ability of DBI.
The figure below illustrates an example of a bromination reaction using DBI.
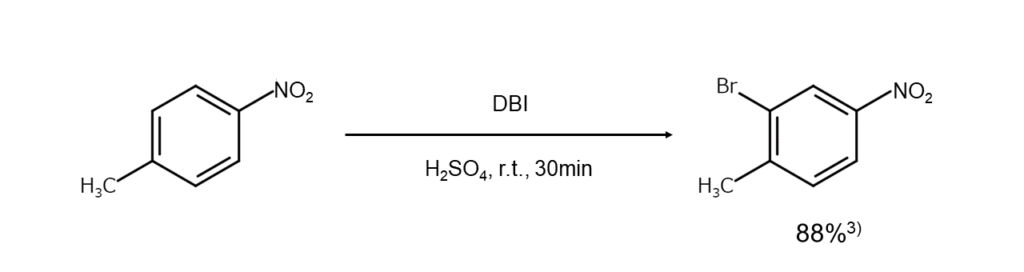
NBS or DBI: Which is more powerful?
You may be wondering which one takes the crown for the higher brominating ability: the subject of this article, DBI, or the most commonly used brominating agent, NBS? To answer this question, let us compare how each agent brominates nitrobenzene as an example.
Using DBI in concentrated sulfuric acid under mild reaction conditions of 20°C for five minutes achieves the bromination of nitrobenzene at an 88% yield.2 While using NBS instead allows for a high bromination yield of 92% with nitrobenzene, NBS requires harsh conditions of 100°C for six hours in the presence of boron trifluoride monohydrate.4
The above results demonstrate how DBI generally exhibits a stronger brominating ability than NBS.
Reference information: Other N-bromo compounds
This series discusses bromination and iodination reactions, which sit at the core of MANAC’s technological capabilities. In addition to this article’s discussion of DBI, we have also covered the following N-bromo compounds:
・N-Bromosuccinimide (NBS)
・1,3-Dibromo-5,5-dimethylhydantoin (DBDMH)
- N-Bromoacetamide (NBA)
There is, in fact, a wide variety of N-bromo compounds that exist beyond the compounds we have covered. The following is a brief overview of a select few.
N-Bromophthalimide
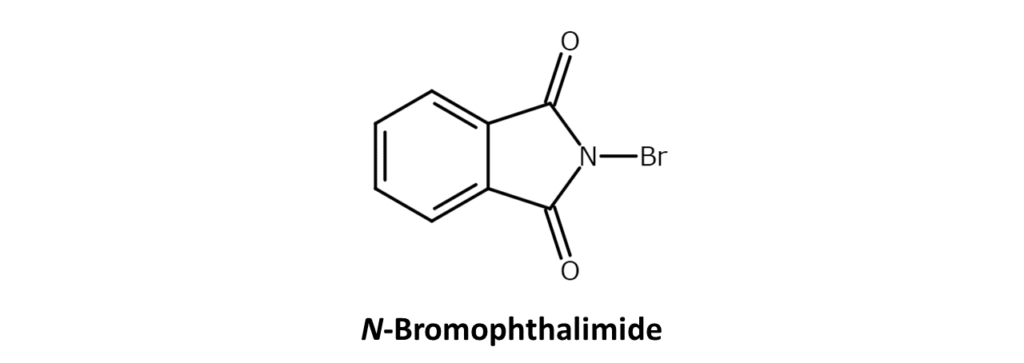
Similar to NBS, it is possible to use N-bromophthalimide for allylic bromination. However, there is little advantage to be gained in using N-bromophthalimide for synthesis due to its low reactivity and tendency to cause phthalimidization during reactions
For example, when N-bromophthalimide is reacted with cyclohexane to produce 3-bromocyclohexene brominated at the allylic position, 2-bromo-1-phthalimidocyclohexane is also generated as an unwanted byproduct.5
N, N, N’, N’-tetrabromobenzene-1,3-disulfonamide (TBBDA)
Poly(N, N’-dibromo-N,N’-(ethylene)benzene-1,3-disulfonamide) (PBBS)
Poly(N,N’-dibromo-N,N’-(1,3-phenylene)benzene-1,3-disulfonamide) (PBPS)
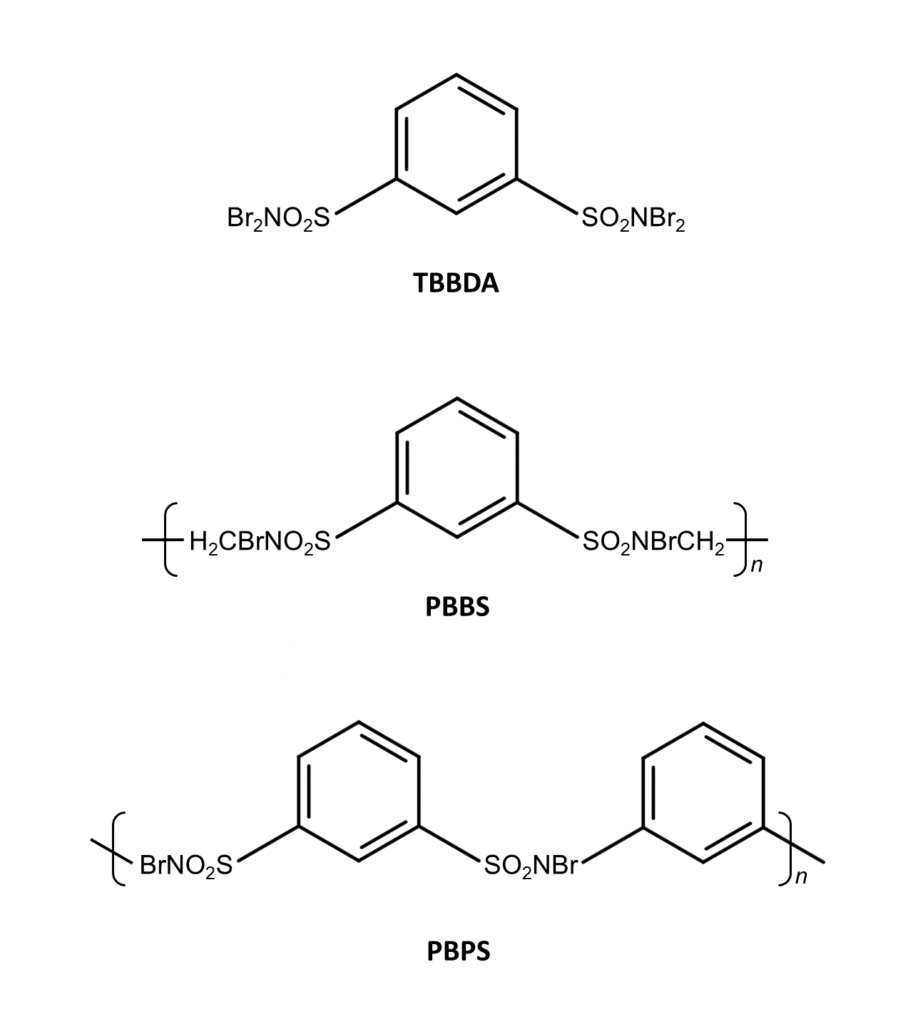
TBBDA, PBBS, and PBPS can be used for selective bromination of the benzylic position, similar to other brominating agents such as NBS and DBDMH. These useful agents are easy to synthesize and are stable under normal storage conditions for two to three months.6
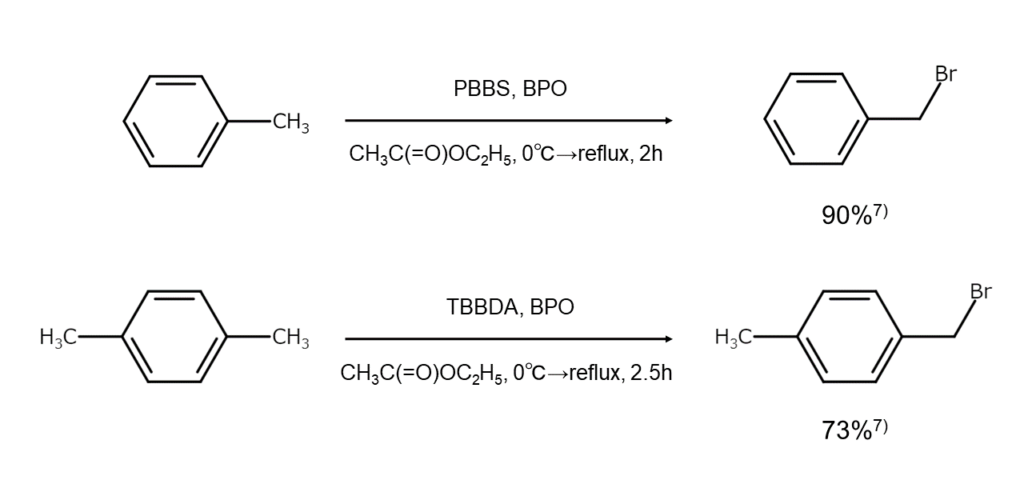
The figures below are examples of bromination reactions using PBBS and TBBDA.
MANAC manufactures and sells NBS, a major N-bromo compound, as well as DBDMH. Please feel free to inquire.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing. (Japanese)
2) Gottardi, W. Monatsh., 1968, 99, 815; Chem. Abstr., 1968, 68, 114222h.
3) Gronowitz, S., Stenhammar, K. et al. Heterocycles, 1981, 15, 947.
4) Takemura, K., Suzuki, T. et al. Chem. Lett., 2003, 32, 932.
5) Ziegler, K., Späth, A. et al. Liebigs Ann. Chem., 1942, 551, 80.
6) Vaghei, R. G., Chegini, M. et al. Tetrahedron Lett., 2009, 50, 1861.
7) Ghorban-Vaghei, R., Chegini, M. et al. Tetrahedron Lett., 2009, 50, 1861.















