A-bromination of carbonyl groups, bromination of carboxylic acids and related compounds: NBS bromination reactions (4): N-bromo compounds (6): Discussion series on bromination/iodination reactions 6
In this series, we discuss bromination and iodination reactions, specialties of MANAC. Various NBS bromination reactions are covered.
In this issue, we review the α-bromination of carbonyl groups and the bromination of carboxylic acids and related compounds. These reactions selectively brominate the alpha position in carbonyl groups and carboxylic acids, and also decarboxylate carboxylic acids to convert them into bromides. We will discuss reaction mechanisms and example reactions. Be sure to use this information as a reference for your research and experiments.
■ What you can learn from this article ✔ By using NBS, selective bromination of the α-position of carbonyl groups and carboxylic acids can be achieved. ✔ NBS can efficiently promote bromination reactions under mild conditions as an alternative to the Hell-Volhard-Zelinsky and Hunsdiecker reactions. ✔ NBS is also suitable for decarboxylation reactions, making it ideal for converting carboxylic acids into bromides or bromoalkenes. ■ Recommended Articles ・ Bromoallene synthesis from propargv alcohol, ortho-selective bromination of aromatic rings: NBS bromination reactions (5): N-bromo compounds (7): Discussion series on bromination/iodination reactions 7 ・ Addition of bromine to alkenes, bromohydrination of alkenes, synthesis of bromoalkanes from alcohols: NBS bromination reactions (3): N-bromo compounds (5): Discussion series on bromination/iodination reactions 5
contents
Describing N-bromosuccinimide (NBS)
A very commonly used, easy-to-handle brominating agent
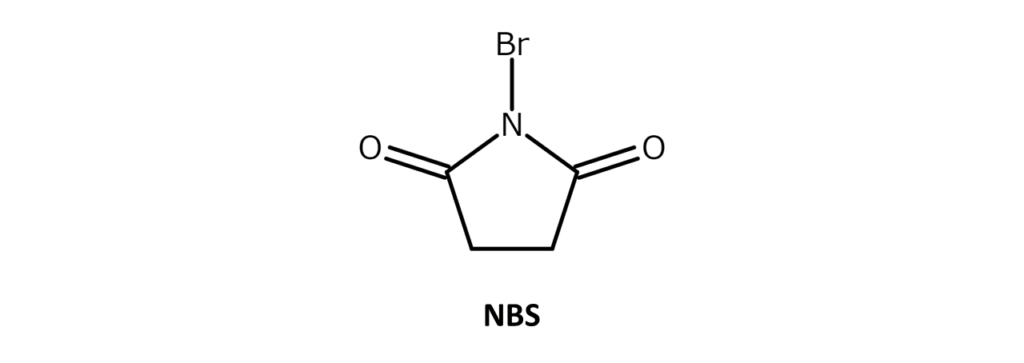
NBS is a white to slightly yellow crystalline powder with a weak bromine odor and a melting point of 173–176°C (decomposition). The compound is soluble in substances such as acetone, THF, DMF, acetonitrile, and DMSO, slightly soluble in water and acetic acid, and has poor solubility in hexane and carbon tetrachloride.
NBS powder is easier to handle than bromine (liquid), making it a common first-line brominating agent in organic syntheses. With its ability to undergo long-term storage in cool, dark, dry places, NBS is also relatively inexpensive.
This article covers information regarding the characteristics and precautions of NBS and gives an overview of bromination reactions that use NBS.
NBS bromination reactions: α-bromination of carbonyl groups
Reaction details
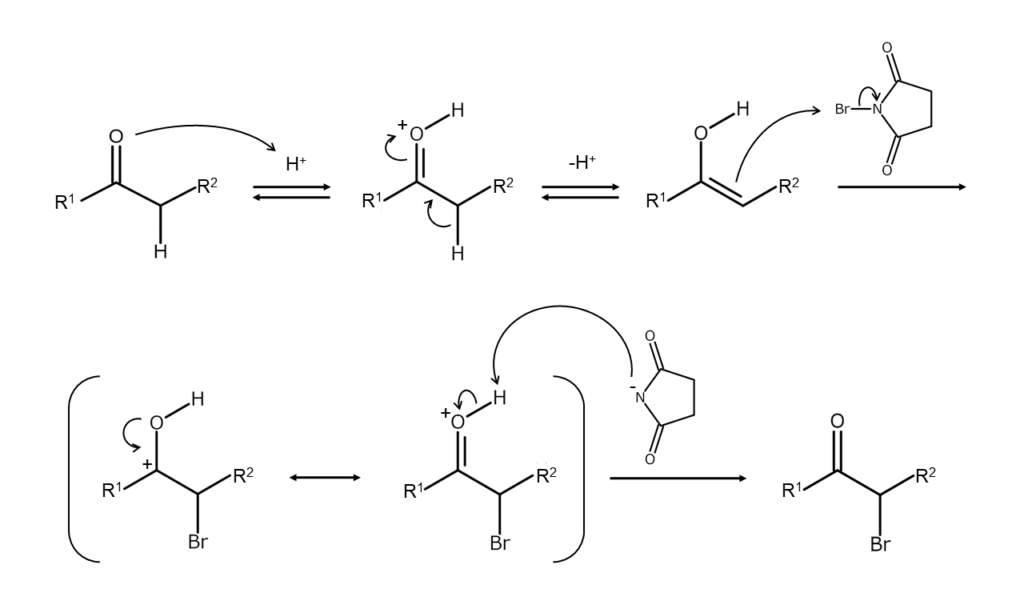
The first reaction brominates carbonyl groups selectively at the alpha position. The mechanism of this reaction differs depending on whether an acid catalyst or a base catalyst is used.
When an acid catalyst is used, the reactant is first enolized. NBS, which acts as an electrophile, then attacks the double bonds and produces an intermediate called halocarbocation. The halocarbocation subsequently undergoes deprotonation, completing the bromination. This reaction normally stops as soon as the first bromine is introduced. Conversely, when a base catalyst is used, the reaction continues until all alpha positions are completely brominated.
The figure below shows the reaction mechanism when using an acid catalyst.
Also, when bromination of α,ß-unsaturated carbonyl compounds is carried out with NBS-(C2H5)3 N⋅3HBr under the presence of potassium carbonate, the alpha position of unsaturated bonds can be selectively brominated.
The figure below illustrates an example of a carbonyl group α-bromination reaction using NBS.
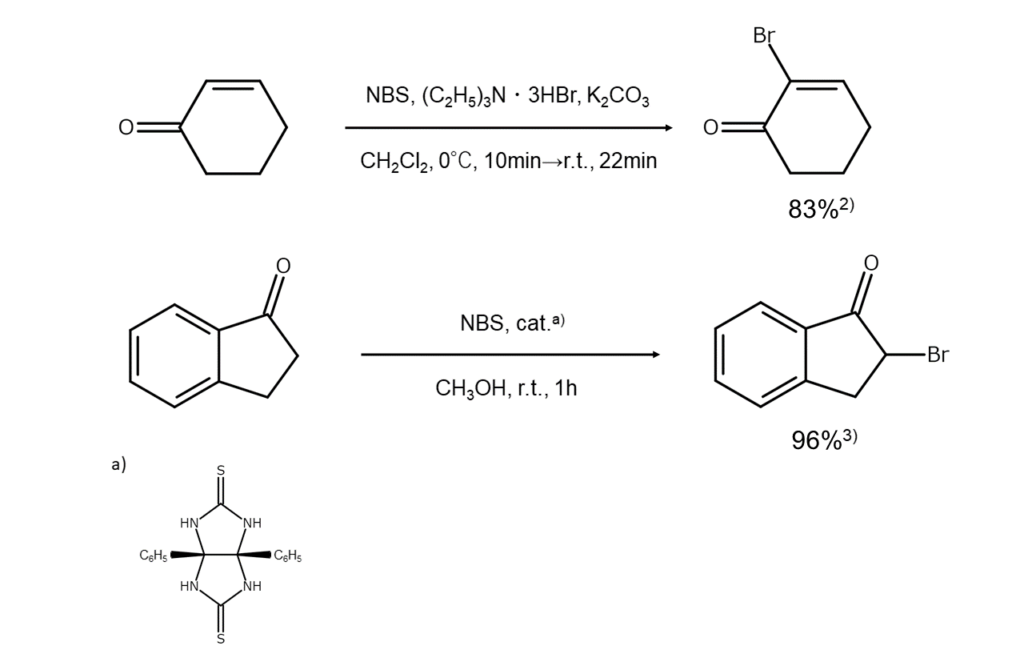
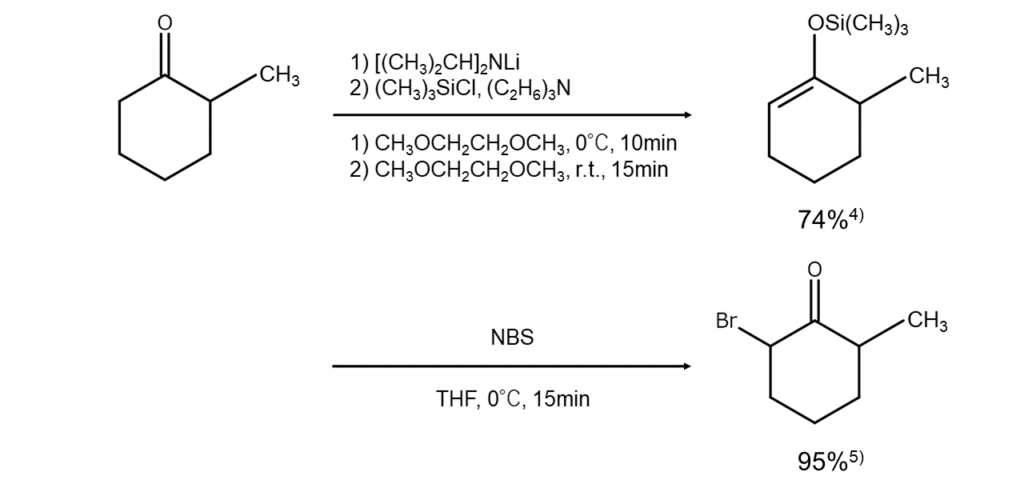
When using asymmetric ketones as reactants, one of the monobromoketones can be specifically synthesized under mild conditions once the reactant is converted into silyl enol ether and the enolization direction is fixed. The figure below illustrates this with an example reaction.
NBS bromination reactions: bromination of carboxylic acids and related compounds
Reaction details
This section reviews methods for the α-bromination of carboxylic acids and the conversion of carboxylic acids into bromides through decarboxylation. Although these reactions can be achieved using methods that do not utilize NBS, using NBS allows the reactions to occur under more favorable conditions. The following section reviews these reactions under traditional methods (non-NBS methods) and NBS-based methods.
(1) Methods to α-brominate carboxylic acids
Traditionally, the Hell–Volhard–Zelinsky reaction6,7,8 has been used to α-brominate carboxylic acids. This method involves slowly adding Br2 to a mixture of carboxylic acid and a small amount of red phosphorus to generate PBr3. The carboxylic acid and PBr3 then react, leading to the generation of acyl bromide, the enolization of the acyl bromide, and the bromination of the resulting enol. Once the reaction is complete, it undergoes hydrolysis to produce an α-bromocarboxylic acid. However, the Hell–Volhard–Zelinsky reaction entails several drawbacks, including that it requires high temperatures and lengthy heat application in addition to having highly variable yield rates.
As a solution to such drawbacks, there is a method to react a carboxylic acid chloride with NBS using a thionyl chloride–carbon tetrachloride–HBr reaction system. This reaction allows for smooth alpha-position bromination under conditions with mild heat application. With its high generality, the reaction is also advantageous because it does not attack the benzylic position.
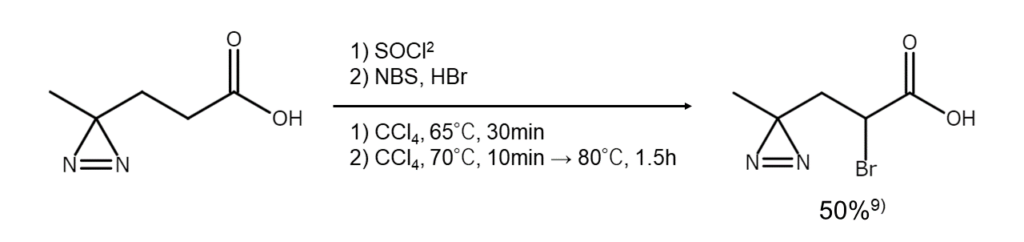
(2) Conversion of carboxylic acids into bromides through decarboxylation
One of the most prominent, long-established methods for converting carboxylic acids into bromides is the Hunsdiecker reaction10, 11. In this reaction, the silver salt of a carboxylic acid is reacted with Br2 to synthesize alkyl bromide. However, carboxylic acids with double bonds or active methylene groups have proven problematic in not easily undergoing this reaction.
Combining NBS with iodosylbenzene or tetrabutylammonium trifluoroacetate (C4H9)4 N + CF3CO2- allows unsaturated carboxylic acids to be efficiently substituted with bromoalkenes. An example of an unsaturated carboxylic acid bromination-decarboxylation reaction using this method is shown below.
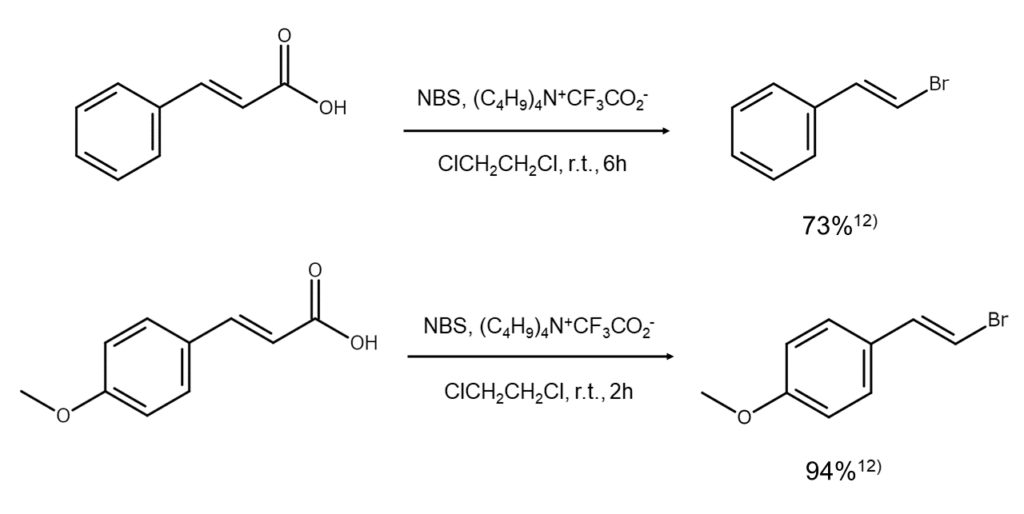
MANAC manufactures and sells NBS and DBDMH, two common N-bromo compounds. Please feel free to inquire.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing.
2) Jyothi, D., HariPrasad, S. Synlett, 2009, 2309.
3) Cao, L., Ding, J. et al. Synlett, 2009, 1445.
4) House, H. O., Czuba, L. J. et al. J. Org. Chem., 1969, 34, 2324.
5) Reuss, R. H., Hassner, A. J. Org. Chem., 1974, 39, 1785.
6) Hell, C. Ber. Dtsch. Chem. Ges. 1881, 14, 891.
7) Volhard, J. Justus Liebig’s Ann. Chem. 1887, 242, 141.
8) Zelinsky, N. Ber. Dtsch. Chem. Ges. 1887, 20, 2026.
9) Ikeda, Y., Behrman, E. J., Synth. Commun. 2008, 38, 2276.
10) Borodine, A. Justus Liebigs Ann. Chem. 1861, 119, 121.
11) Hunsdiecker, H. et al., Ber. Dtsch. Chem.Ges. B 1942, 75, 291.
12) Naskar, D., Roy, S. Tetrahedron 2000, 56, 1369.