
Heterocyclic compound iodination overview and reactions: Discussion series on bromination/iodination reactions 23
So far, several articles in this series have discussed aromatic compound and aliphatic
compound iodination reactions that use elemental iodine. Through these articles, it has
been our aim to help readers gain a systematic understanding of the characteristics and
precautions, as well as the advantages and disadvantages, of using elemental iodine for
these purposes.
This time, we will discuss our final topic of iodination reactions using elemental iodine.
The final topic is none other than heterocyclic compound iodination. In this article, we
explain various reaction examples based on reports. Be sure to use this information as a
reference for experiments.
contents
Describing heterocyclic compound iodination with elemental iodine
Heterocyclic compounds encompass a wide variety of compound structures, including
oxygen-containing heterocyclic compounds commonly found as furans and pyrans, sulfur-
containing heterocyclic compounds such as thiophenes, and nitrogen-containing
heterocyclic compounds that include azoles and azines. Due to wide structural variations
and an equally diverse range of reactivity, the iodination of these compounds cannot be
discussed as a whole.
For this reason, we will focus on five-membered and six-membered heterocyclic
compounds commonly used in the laboratory and give examples of common reactions.
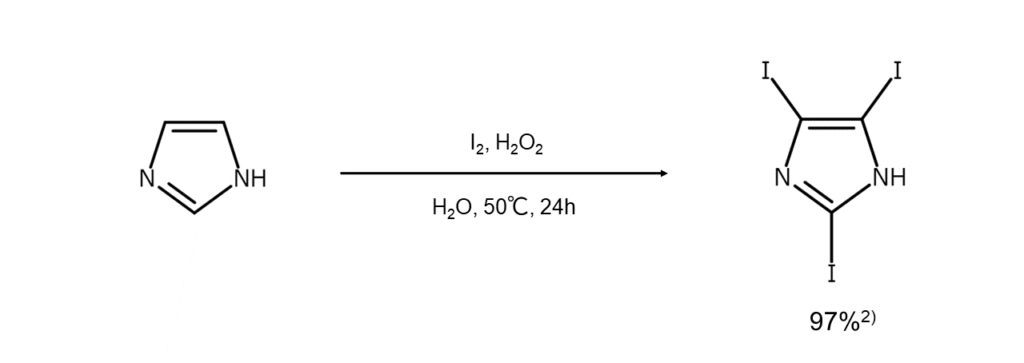
Reaction Example 1: An efficient and eco-friendly synthesis method using hydrogen
peroxide
Reacting a five-membered heterocyclic compound with elemental iodine in water in the
presence of hydrogen peroxide results in exceptionally high iodide yields 2 . This method is
efficient and eco-friendly, and we can expect to see applications for the reaction products
ranging from organic synthesis to pharmaceuticals.
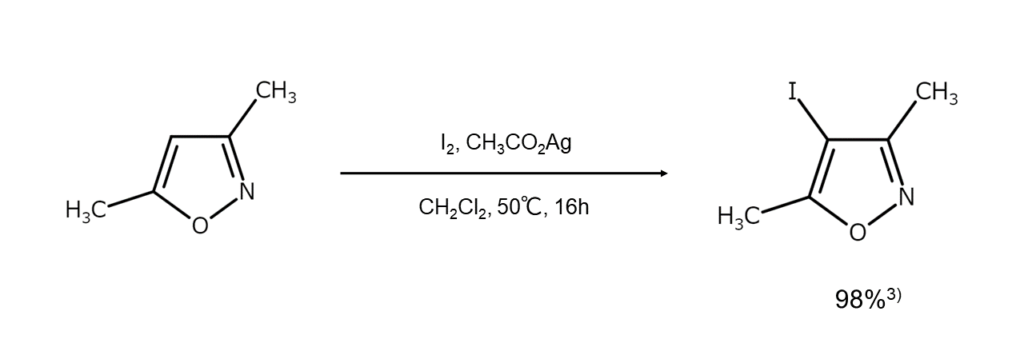
Reaction Example 2: A synthesis method for obtaining iodides suitable as carbene
complex precursors
This method allows for the selective iodination of various heterocyclic compounds by
reacting elemental iodine with silver acetate in dichloromethane 3 . The reaction takes place
under mild conditions and is regarded as a low-cost, efficient approach to iodination. The
reaction products are suitable precursors to N-heterocarbene complexes.
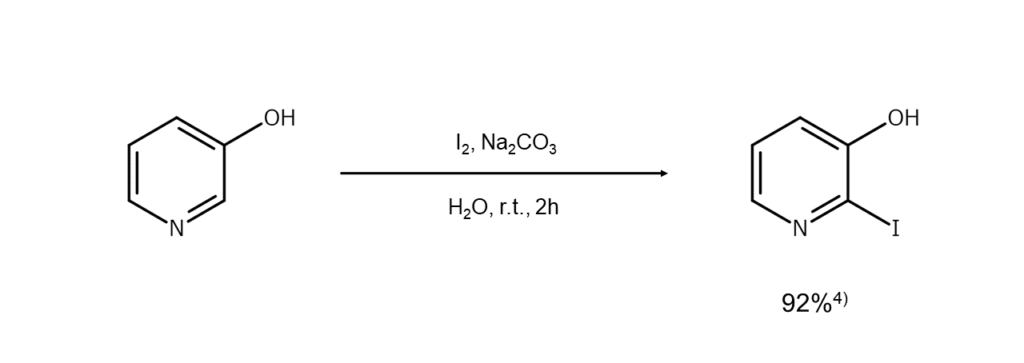
Reaction Example 3: A reaction method with exceptionally mild conditions
This method involves reactions in the presence of sodium carbonate under mild
conditions – namely, two hours at room temperature. Loidreau et al. 4 use this type of
reaction as a starting reaction when synthesizing compounds that substitute the pyrimidine
ring 4-position with a primary or secondary amino group (4-aminopyrido[2’,3’:4,5]furo[3,2-
d]pyrimidines).
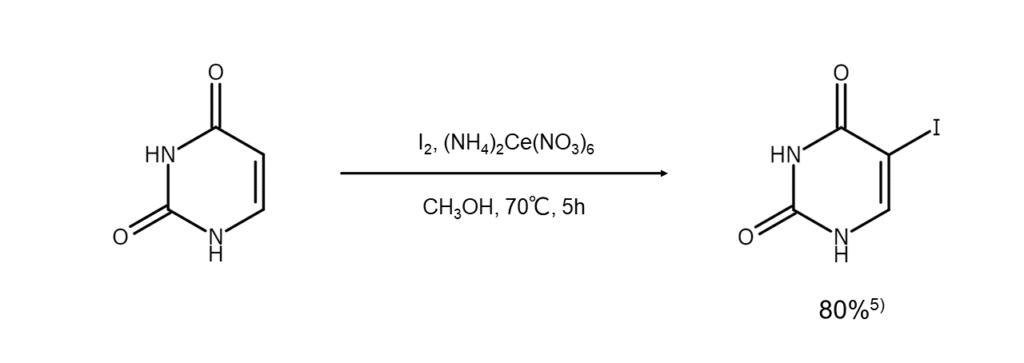
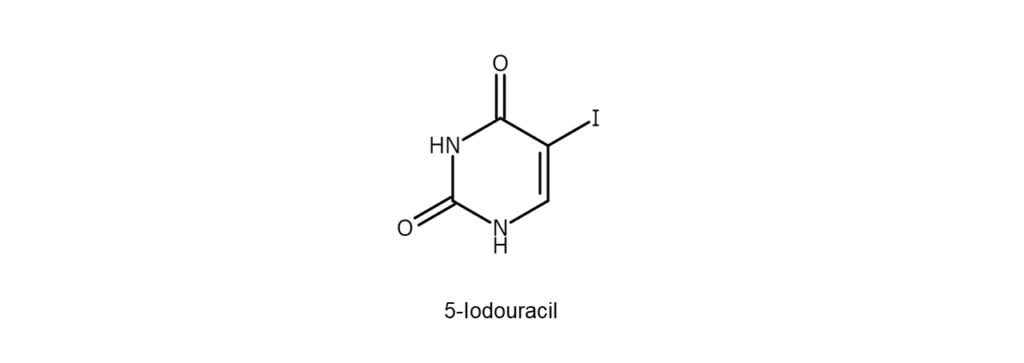
Reaction Example 4: Uracil iodination method
This reaction iodinates uracil, one of the nucleobases comprising RNA 5 . Reacting elemental
iodine with uracil in the presence of ammonium cerium(IV) nitrate will result in the iodination
of uracil at the 5-position, which generates 5-iodouracil.
Column: The radiation-enhancing effects of iodouracil
Did you know that the reaction product compound discussed in Reaction Example 4 above,
5-iodouracil, is used in cancer treatment?
One of the options available for cancer treatment is radiotherapy. Radiotherapy exposes
the part of the body affected by cancer to radiation, such as x-rays, electron rays, and
gamma rays, damaging the DNA of the cancer cells and causing them to die. This form of
treatment is possible because cancer cells are more susceptible to radiation damage than
non-cancer cells, which enables radiation to be applied while minimizing adverse effects on
healthy cells.
In order to improve the effectiveness of radiotherapy, the sensitivity of cancer cells to
radiation must be augmented. Enter the radiosensitizer, a vital component of radiotherapy.
5-Iodouracil, discussed above, is actually also known for its properties as a radiosensitizer.
In 2016, a research team that included members from Kyoto University and Tohoku
University released a study demonstrating the mechanism behind the ability of 5-iodouracil
to absorb x-rays and subsequently create multiple high-energy ions as well as low-energy
electrons when exposed to intense x-ray pulses. The press release for this research 6
describes how the study uncovers the radiosensitizing mechanisms of 5-iodouracil at the
molecular level.
This research is another example of how widely iodides are used throughout many fields.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of
Bromine & Iodine Compounds”. Maruzen Publishing. (Japanese)
2) Gallo, R. D. C., Ferreira, I. M. et al. Tetrahedron Lett., 2012, 53, 5372.
3) Iglesias, M. et al. Tetrahedron Lett., 2010, 51, 5423.
4) Loidreau, Y. et al. Tetrahedron Lett., 2012, 53, 944.
5) Asakura, J., Robins, M. J. J. Org. Chem., 1990, 55, 4928.
6) Kyoto University. “Ultrafast Dynamics of a Nucleobase Analogue Illuminated by a Short
Intense X-ray Free Electron Laser Pulse”. (2016).
https://www.kyoto-u.ac.jp/ja/research-news/2016-07-04-0













