
Bromination reactions with hydrogen bromide (bromomethylation of alcohols, phenols, and aromatic rings): Hydrogen bromide (4): Discussion series on bromination/iodination reactions 37
In this series, we discuss bromination and iodination reactions, specialties of MANAC. In the previous article, we explained hydrogen bromide additions to alkenes and alkynes. Our review illustrated the great convenience offered by these methods, which yield different products from the same substrate simply by changing reaction conditions.
This time, we focus on using hydrogen bromide in the bromomethylation of alcohols and phenols, as well as in the bromomethylation of aromatic rings. While both are exceptional methods that provide high yields of target products, care is required as there is a limited selection of substrates that can be used effectively in some cases.
This article gives an overview of the basic principles, characteristics, and precautions of these reactions. We recommend reading the whole article to gain more knowledge toward achieving success in the lab.
contents
Bromination reactions with hydrogen bromide: Bromomethylation
① Ortho-bromomethylation of alcohols and phenols
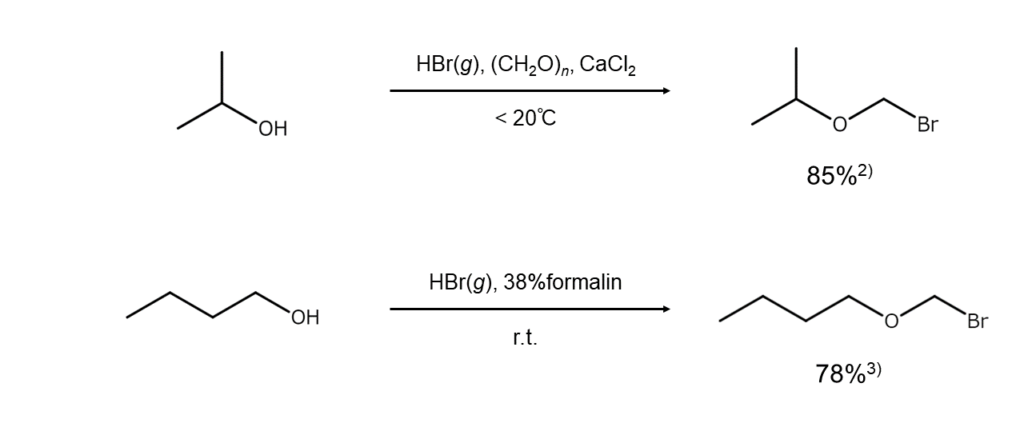
High yields offered by the ortho-bromomethylation of alcohols
After suspending paraformaldehyde ((CH2O)n) in an alcohol (ROH), ortho-bromomethylation occurs by passing dried hydrogen bromide (HBr) through, resulting in high yields of the corresponding bromomethyl ether (ROCH2Br).
Lower bromomethyl ethers generated from lower alcohols will fume in humid air, and distillation attempted at normal pressure will result in decomposition. Caution is required when handling lower bromomethyl ethers as these compounds have strong lachrymatory properties and cause irritation of the skin and mucous membranes. On the other hand, bromomethyl ethers from higher alcohols can be handled stably.
The figures below are examples of ortho-bromomethylation reactions of alcohols.
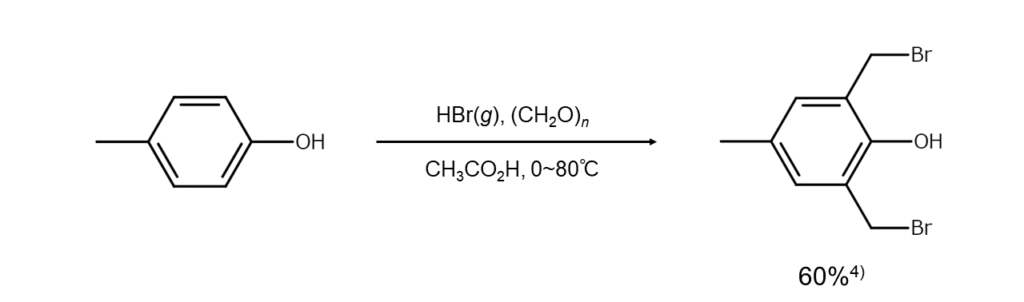
Caution required for the ortho-bromomethylation of phenols
As with alcohols, phenols can also undergo ortho-bromomethylation. However, in the case of phenols activated by the introduction of a substituent or by other means, aromatic ring bromomethylation will occur preferentially. Therefore, care must be taken in the reaction design.
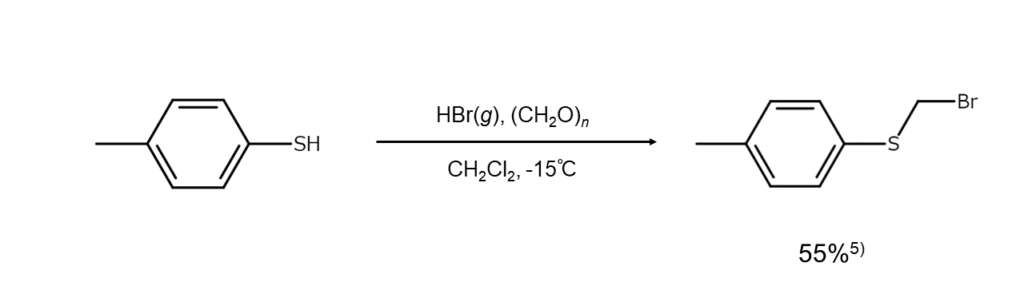
Thiols give similar reactions
Thiols (RSH), which are analogous to alcohols (the position occupied by an oxygen atom in alcohols is occupied by a sulfur atom in thiols), will undergo bromomethylation similarly to alcohols to produce bromomethyl sulfides (RSCH2Br).
The following is an example of the bromomethylation of thiophenol.
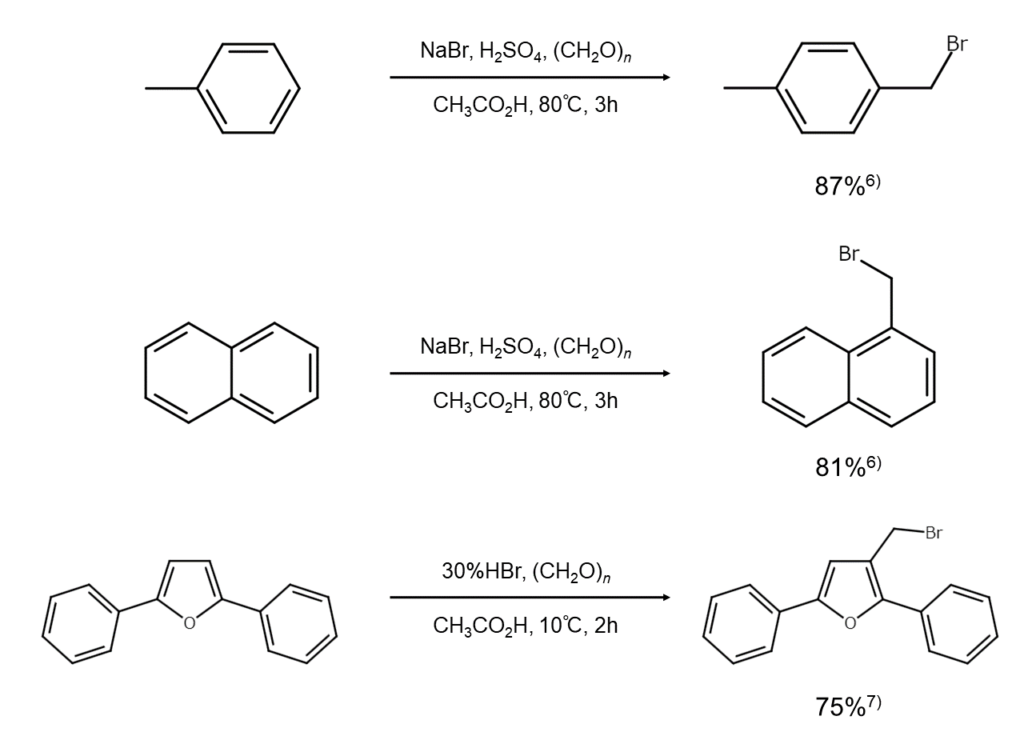
② Aromatic ring bromomethylation
Producing benzyl bromide from hydrogen bromide and formaldehyde
Reacting hydrogen bromide and formaldehyde with an aromatic hydrocarbon will cause aromatic ring bromomethylation, producing relatively high yields of the corresponding benzyl bromide. This method is useful in cases where the targeted benzyl bromide cannot easily be obtained through methods of radically brominating the side chain of a methylarene using bromine.
While paraformaldehyde ((CH2O)n) is generally used as the source of formaldehyde, other possible sources include formalin (the aqueous solution of formaldehyde commercially available as preparations of 30-35% concentration), trioxane, and dimethoxymethane. Further, hydrobromic acid or sodium bromide (NaBr) with concentrated sulfuric acid are commonly used as alternatives to hydrogen bromide. In cases of slow reaction progression, zinc chloride (ZnCl2), tin(IV) chloride (SnCl4), aluminum chloride (Al2Cl6), phosphoric acid, sulfuric acid, or other acid catalysts are added.
The following are examples of aromatic ring bromomethylation reactions.
Reaction mechanisms
Aromatic ring bromomethylation occurs through the following mechanism.
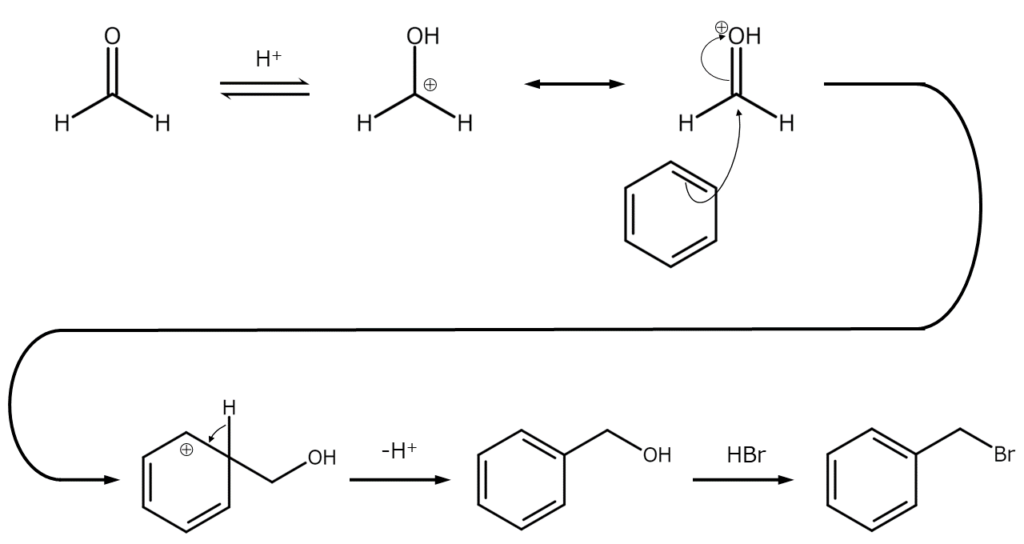
Since the reaction occurs via benzyl alcohol generation, if the hydrogen bromide concentration level is low, diarylmethane is generated as the main product of benzyl alcohol and aromatic ring reactions. Additionally, because benzyl bromides are highly reactive, diarylmethane will occur more readily when the reaction takes place in high temperatures.
A limited selection of effective substrates
Aromatic ring bromomethylation, a focus of this article, differs from the long-established and well-known chloromethylation known as the Blanc-Quelet reaction in terms of the currently limited substrate range that can be used effectively.
For example, as explained in the “Ortho-bromomethylation of alcohols and phenols” section, phenols and thiophenols undergo bromomethylation at the hydroxy group or thiol group rather than the aromatic ring. Furthermore, nitrobenzenes, benzoic acids, and other compounds containing strong electron-withdrawing groups will not react. As phenols and anilines with strong electron-donating groups will readily undergo resinification under these conditions, it is necessary to protect the functional groups using acetyl groups in order to lower the reactivity to an appropriate level.
Column: Convenient yet hazardous bromomethylating reagents: 4-Chlorobutyl (bromomethyl) ether
The “② Aromatic ring bromomethylation” section of this article explains the bromomethylation of aromatic compounds using formaldehyde and hydrogen bromide. However, there exists another method of aromatic compound bromomethylation. The method came about through a rather unexpected clue gained from chloromethylation.
Reagents known as chloromethyl ethers have long been used for the chloromethylation of aromatic compounds. Some may then wonder, “If that’s the case, then bromomethylation should be possible using bromomethyl ethers.” However, due to their instability and difficulty in handling, bromomethyl ethers have not been useful on their own for bromomethylation.
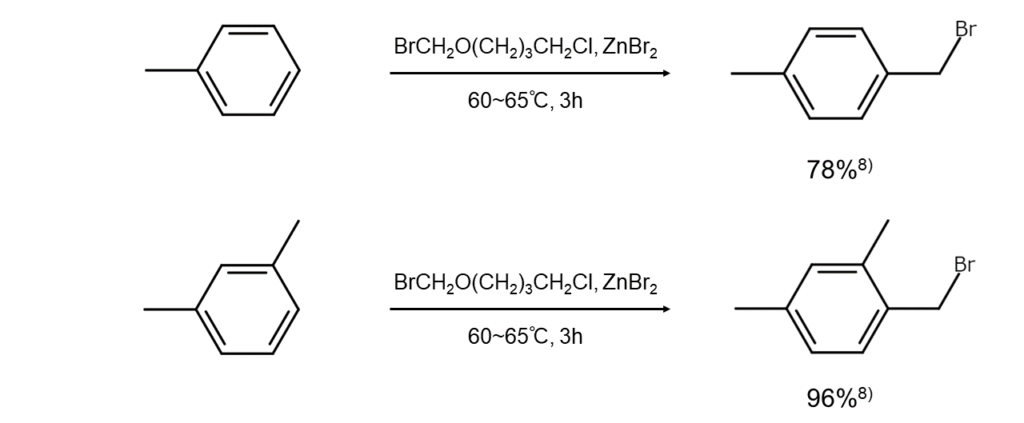
4-Chlorobutyl (bromomethyl) ether (1-bromomethoxy-4-chlorobutane) was then devised to address the shortcomings of bromomethyl ethers. This reagent enables aromatic hydrocarbons to readily undergo bromomethylation in the presence of Lewis acid catalysts, such as zinc bromide (ZnBr2), SnCl4, and titanium tetrachloride (TiCl4). Advantages of the reagent include its state as a liquid under normal temperature and pressure and its low vapor pressure that minimizes irritation to the eyes and throat.
Regardless, certain aspects do require care. Similar to chloromethyl ethers, 4-chlorobutyl (bromomethyl) ether has been identified as carcinogenic. Therefore, the reagent should be used in a fume hood with proper ventilation.
See below for examples of aromatic ring bromomethylation using 4-chlorobutyl (bromomethyl) ether.
Column: Benzyl bromides in polymer modification
Benzyl bromides appear in the “② Aromatic ring bromomethylation” section of this article as main reaction products. Did you know that such benzyl bromides are also used to modify polymers?
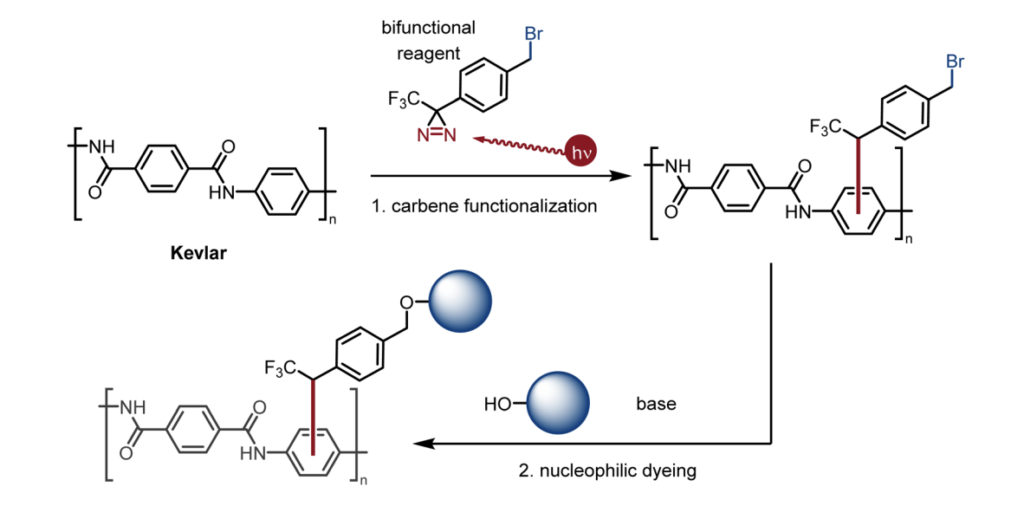
Richard et al. report on a bifunctional diazirine reagent containing a benzyl bromide group applied as a reagent for photochemically dyeing inert polymer materials used in bulletproof vests and other such applications, including the para-aramid synthetic fiber Kevlar.9)
The reagent is activated by UV irradiation and bonds to the fiber molecules. The nucleophilic dyes then attack the benzyl bromide group, causing a substitution reaction that bonds the dyes to the fiber molecules. This dyeing process can be carried out under mild conditions and produces dyed fabric with colors resistant to fading even when washed. Perhaps we will see a growing selection of bulletproof vest color offerings in the future thanks to the power of benzyl bromides.
MANAC is a global leader in bromination and iodination reactions. Please inquire with us about any bromination or iodination needs.
References
1) MANAC Inc. Research Lab., Suzuki, H. (ed.). Shuuso oyobi Youso Kagoubutsu no Yuuki Gousei: Shiyaku to Gouseihou [Organic Syntheses of Bromine & Iodine Compounds: Reagents & Synthesis Methods], Maruzen Publishing (2017).
2) Lucien, H. W., Mason, C. T. J. Am. Chem. Soc., 1949, 71, 258.
3) Blair, C. M., Henze, J. Am. Chem. Soc., 1932, 54, 399.
4) Bright, W. M., Cammarata, P. J. Chem. Soc., 1952, 74, 3690.
5) Bredereck, H., Bäder, E. et al., Chem. Ber., 1954, 87, 784.
6) Kubiczek, G., Neugebauer L. Monatsh., 1950, 81, 917.
7) Bailey, P. S., Smith, J. C. J. Org. Chem., 1956, 21, 628.
8) Olah, G. A., Beal, D. A. et al. Synthesis, 1974, 560.
9) Richard, Y. L., Shao-Xiong, L. L. et al. Polym. Chem., 2023, 14, 4205.













