
Bromination reactions with hydrogen bromide (additions to alkenes/alkynes): Hydrogen bromide (3): Discussion series on bromination/iodination reactions 36
In this series, we discuss bromination and iodination reactions, specialties of MANAC.
This issue reviews the addition of hydrogen bromide to alkenes and alkynes. These reactions are exceptionally useful since they allow different products to be obtained using the same substrate simply by changing the conditions. However, some products have a lower regioselectivity, and in some cases, better results may be achieved by using catalysts. Therefore, it is important to pay attention to the reaction design.
In this article, we give an overview of the basic principles, characteristics, and precautions of these reactions. Be sure to read to the end to learn about leveraging these valuable reactions.
contents
Bromination reactions with hydrogen bromide: Additions to alkenes
Achieving either ionic reactions or radical reactions based on reaction conditions
Adding hydrogen bromide to an alkene at room temperature will generate a bromoalkane. However, the reaction mechanisms involved differ greatly depending on the reaction conditions.
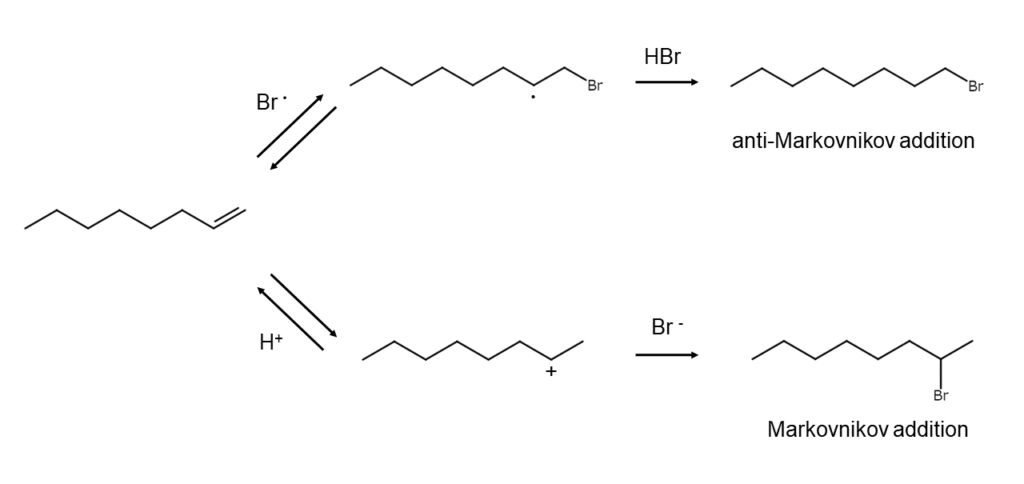
When the reaction occurs in a polar solvent, it takes place as an ionic reaction. The H+ generated from hydrogen bromide is added to the double bond of the alkene to generate carbocation, which is then captured by the bromide ion (Br–), yielding the addition product. Since the reaction proceeds via carbocations, the addition direction follows Markovnikov’s rule and prioritizes bromoalkane generation in the following order: tertiary > secondary > primary.
By contrast, when the reaction occurs in a non-polar solvent in the presence of a peroxide or with the application of light, it takes place as a radical reaction, which is significantly faster than an ionic reaction. Depending on the initiator, the bromine atoms (Br•) that occur are added to the alkene to generate bromoalkyl radicals, which then extract hydrogen from the hydrogen bromide to yield the addition product. Since these reactions take place via bromoalkyl radicals that are more stable, these radical reactions are characterized by an addition direction opposite to the ionic reactions (anti-Markovnikov additions).
Since the addition reaction speed of electron-deficient alkenes is slow, Fe2Cl6, Fe2Br6, Al2Cl6, or other Lewis acids can be used side-by-side to promote reactions.
The following are examples of bromoalkane synthesis achieved through the addition of hydrogen bromide to alkenes.
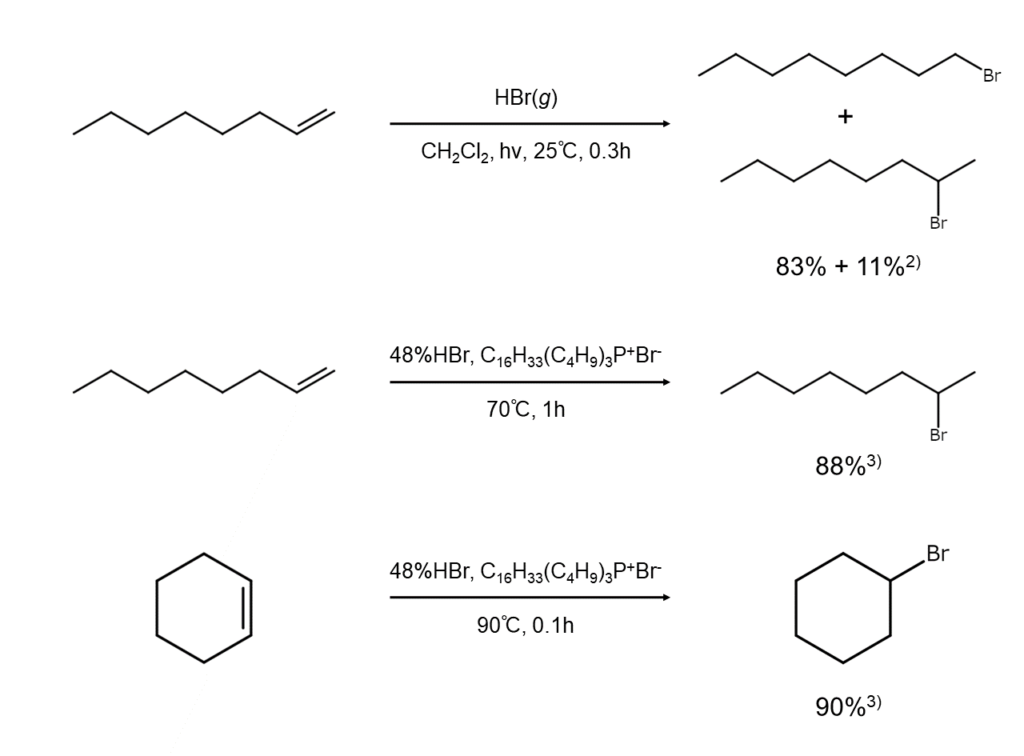
Degree of regioselectivity dependent on reaction conditions and substrates
Using hydrogen bromide to conduct bromine additions to alkenes is a convenient approach to synthesis since different bromoalkanes can be obtained from the same substrate and reagent simply by changing the reaction conditions. However, the regioselectivity explained above is not complete regioselectivity. For example, in the case of anti-Markovnikov additions, there are instances where satisfactory regioselectivity cannot be achieved.
In other cases, however, the regioselectivity of the reaction can grow exceptionally high. For example, the addition of bromine atoms to alpha, beta-unsaturated carbonyl compounds and alpha, beta-unsaturated nitriles always occurs at the beta position, yielding beta-bromoketones or bromonitriles as single products.
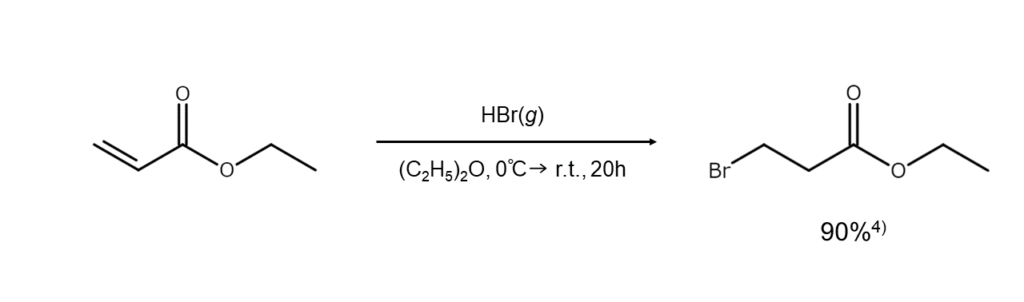
When aiming to obtain a specific product, it is necessary to carefully consider the substrate and reaction conditions to achieve a high degree of regioselectivity.
Bromination reactions with hydrogen bromide: Additions to alkynes
Addition reactions to alkynes slower than to alkenes
As acetylene carbons are sp-hybridized, they have a greater s-character than olefin carbons, making them less prone to protonation. In turn, hydrogen bromide (HBr) additions to alkynes are generally slow. For this reason, reactions are usually carried out using copper(I) bromide (CuBr) or tetra-alkylammonium bromides (R4N+Br–) as catalysts.
As with alkenes, alkynes exhibit differences in terms of HBr addition direction between ionic reactions and radical reactions. However, these differences are not as pronounced as they are for alkenes. In addition, when HBr is present in excess, a double addition of HBr will occur, yielding gem-dibromo compounds.
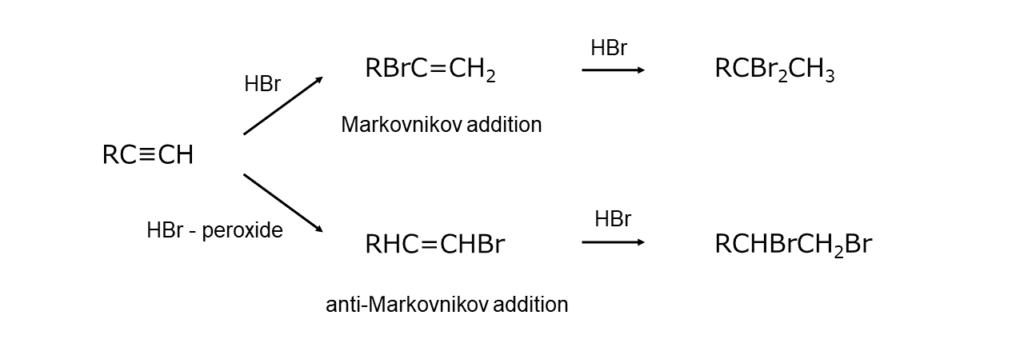
The following examples illustrate bromoalkene synthesis resulting from HBr additions to alkynes.
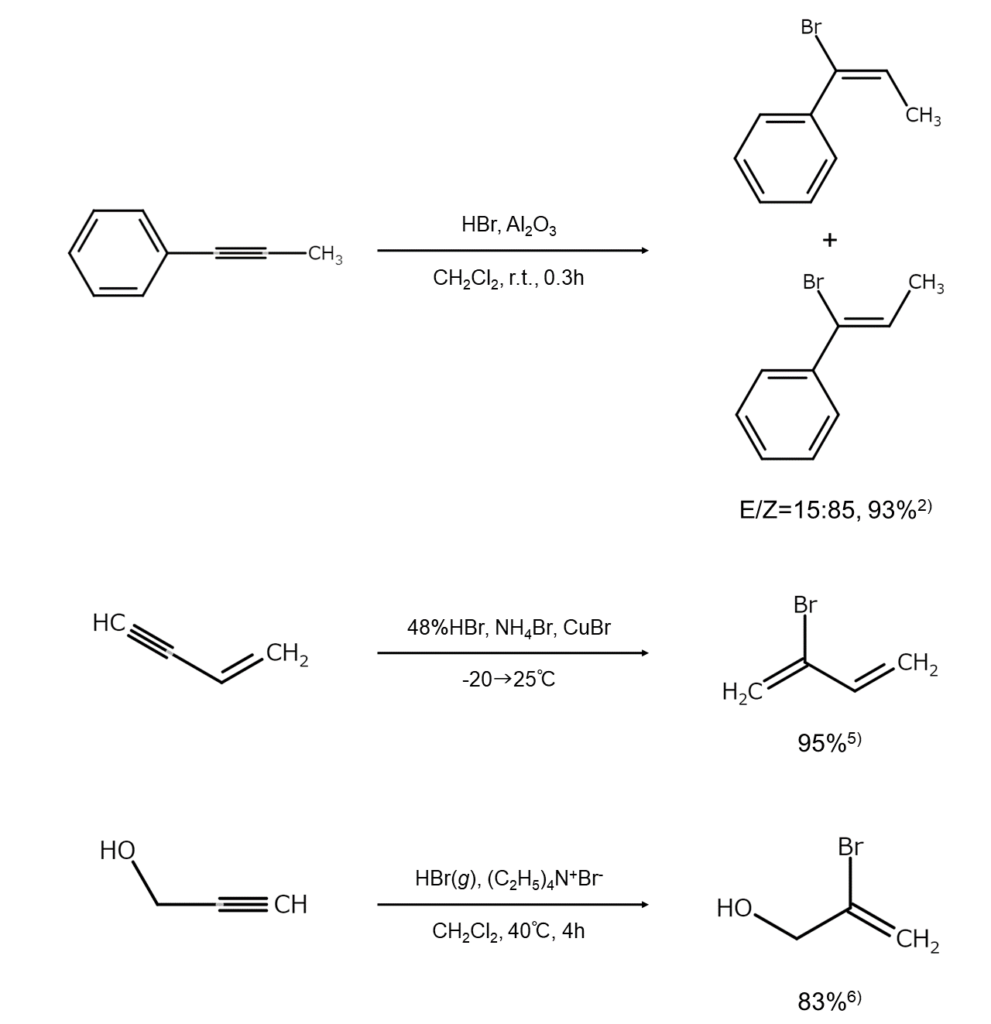
Column: Reacting just like an alkene – A glance at HBr addition reactions to cyclopropane
So far in this article, we have covered HBr addition reactions to alkenes and alkynes, both of which are unsaturated hydrocarbons. The explanations are provided to help readers gain an understanding of selecting reaction conditions, using catalysts, and precautions.
With these basics in mind, let’s take a look at a compound that accepts HBr additions like an unsaturated hydrocarbon despite not being one. That compound is cyclopropane, a saturated hydrocarbon with a three-membered ring.
The cyclopropane three-membered ring is structured with what is known as “banana bonds” in reference to the bent banana-like shape of the carbon-carbon bonds. The structure gives cyclopropane a certain degree of pi bondability, differentiating it from common cycloalkanes comprised solely of sigma bonds. As a result, cyclopropane exhibits properties similar to alkenes, readily undergoing ring-openings through HBr actions and converting into a bromoalkane. There are no other cycloalkanes that can undergo such reactions.
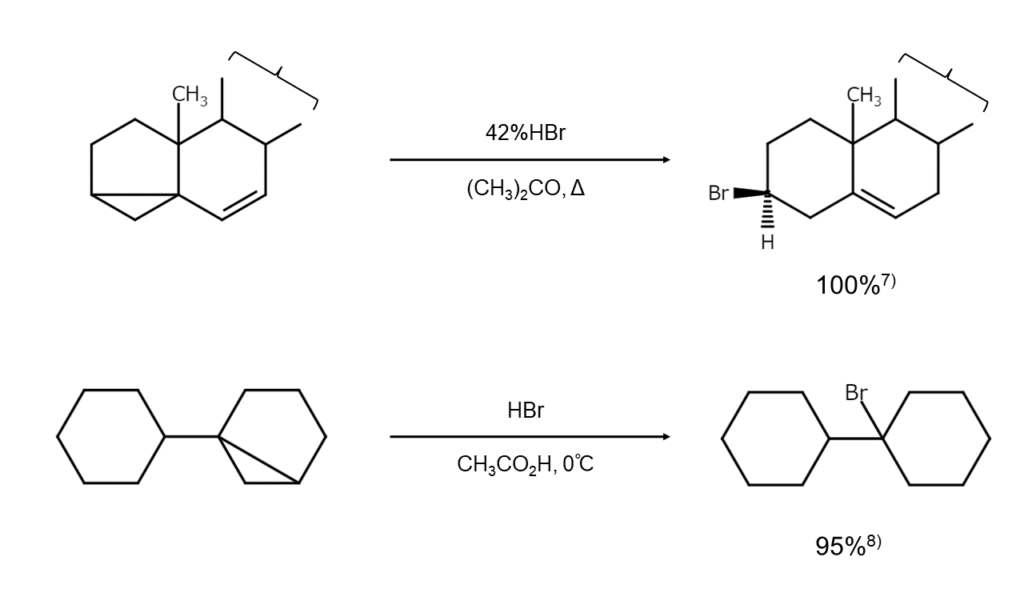
Lastly, let’s touch upon precautions. The large bends in the cyclopropane structure make the compound chemically unstable and highly reactive. It is crucial to exercise the utmost caution to control reactions when using HBr to treat compounds containing the cyclopropane structure.
MANAC is a global leader in bromination and iodination reactions. Please inquire with us about any bromination or iodination needs.
References
1) MANAC Inc. Research Lab., Suzuki, H. (ed.). Shuuso oyobi Youso Kagoubutsu no Yuuki Gousei: Shiyaku to Gouseihou [Organic Syntheses of Bromine & Iodine Compounds: Reagents & Synthesis Methods], Maruzen Publishing (2017).
2) Kropp, P. J., Daus, K. A. et al. J. Am. Chem. Soc., 1990, 112, 7433.
3) Landini, D., Rolla, F. J. Org. Chem., 1980, 45, 3527.
4) Mozingo, R., Patterson, L. A. Org. Synth. Coll. Vol. III, 576 (1955).
5) Keegsta, M. A., Verkruijsse, H. D. et al. Synth. Commun. 1991, 21, 721.
6) Cousseau, J. Synthesis, 1980, 805.
7) Riegel, B., Hager, G. P. et al. J. Am. Chem. Soc., 1946, 68, 2562.
8) Läber, G. Liebigs Ann. Chem., 1954, 588, 79.













