
Overview of bromination reactions with phosphorus bromides/Bromination reactions that use phosphorus(III) bromide (PBr3): Phosphorus bromides (1): Discussion series on bromination/iodination reactions 39
In this series, we discuss bromination and iodination reactions, specialties of MANAC. This article launches our exploration of bromination reactions that use phosphorus bromides.
The term “phosphorus bromide” encompasses a great variety, ranging from phosphorus(III) bromide to phosphorus(V) bromide, as well as phosphoryl bromide and beyond. Systematically mastering the series of bromination reactions that use each of these reagents will certainly prove to be of tremendous use for experiments and research.
In this article, we first give a simple digest of bromination reactions with various phosphorus bromides. We then explain reactions that use phosphorus(III) bromide in more detail.
contents
Overview of bromination reactions with phosphorus bromides
Upcoming articles in this series will discuss bromination reactions with the reagents listed below.
Phosphorus(III) bromide (PBr3)
This reagent is often used in reactions that synthesize bromoalkanes from alcohols. PBr3 offers advantages over hydrobromic acid for reactions, including a lower tendency toward isomerization and elimination reactions.
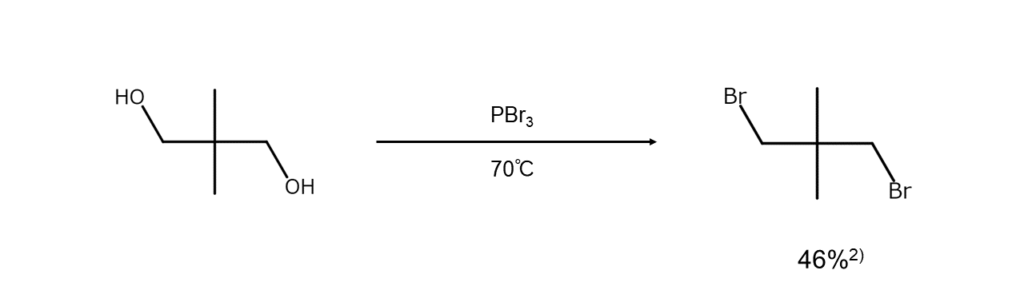
Bromine and phosphorus
Here, we refer to a method of reacting bromine with phosphorus in an alcohol and utilizing the generated PBr3 in situ. Using this method allows for lower costs than using commercially available PBr3.
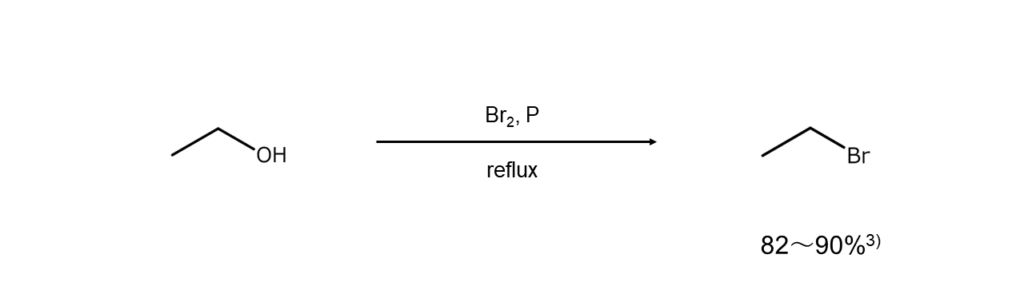
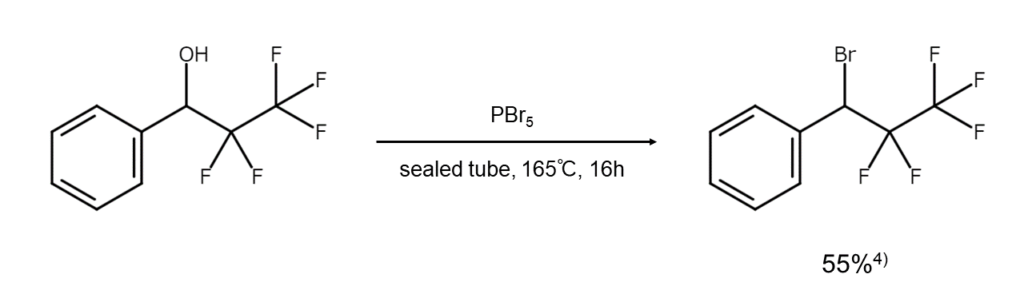
Phosphorus(V) bromide (PBr5)
PBr5 is often used in reactions that substitute the hydroxy groups of an aromatic ring or heteroaromatic ring with bromine atoms, as well as in reactions to convert aldehydes into 1,1-dibromoalkanes. It is also useful for alcohols that do not react effectively with PBr3 when converting them into bromides.
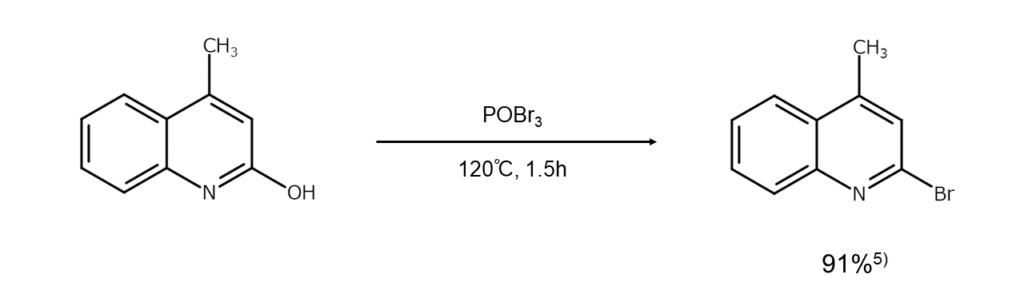
Phosphoryl bromide (POBr3)
POBr3 is used when substituting the hydroxy groups of aromatic rings and heterocycles with bromine atoms. As its bromination capacity is not as high as PBr5, POBr3 can be described as a safe reagent.
Bromo-phosphoranes
Dibromo-triphenyl-phosphorane and 1,2-bis(dibromo-diphenyl-phosphoranyl)ethane are used as brominating reagents. They are useful in reactions that synthesize bromoalkanes from alcohols or bromoarenes from phenols.
Bromination reactions with phosphorus bromides: Reactions that use phosphorus(III) bromide as a reagent
Phosphorus(III) bromide basics
Phosphorus(III) bromide (PBr3) is a colorless liquid with a harsh odor. It is highly corrosive and will damage many metal products in the presence of moisture. Caution is required when handling as PBr3 produces heavy, toxic fumes and is severely harmful to the skin and mucous membranes.
PBr3 readily dissolves in certain compounds such as ether, acetone, dichloromethane, trichloromethane, carbon disulfide, and benzene. When combined with an alcohol, PBr3 will react violently, generating HBr. While PBr3 can undergo stable storage in a dry state, exposure to moisture will generate HBr and convert it into phosphoryl bromide (POBr3). In water, PBr3 undergoes rapid hydrolysis and becomes phosphorous acid and hydrobromic acid.
Reactions 1: Synthesizing bromoalkanes from alcohols
Reaction mechanisms
While hydrobromic acid is well-known as a reagent for synthesizing bromoalkanes from alcohols, using PBr3 instead can further reduce the frequency of isomerization and elimination reactions. That is why using PBr3 is advantageous when converting secondary and tertiary alcohols into bromoalkanes. During these processes, optically active alcohols yield optically active bromoalkanes of inverted configurations.
Bromination reactions with PBr3 occur in the two following stages, as shown below: (1) Alcoholysis that forms phosphite esters and their precursors (Formula 1 below), and (2) Simultaneously generated HBr resulting in the brominolysis of Stage (1) products (Formula 2 below).
Formula 1: ROH + PBr3 → Br2POR, BrP(OR)2, P(OR)3 + HBr
Formula 2: Br2POR, BrP(OR)2, P(OR)3 + HBr → RBr + H3PO3
The reaction above requires 0.33–0.40 equivalents of PBr3 for each individual hydroxy group. While a low PBr3 ratio will result in significant amounts of organophosphorus compound byproducts (Br2POR, BrP(OR)2, P(OR)3), further treating these crude products with concentrated hydrobromic acid can improve yields.
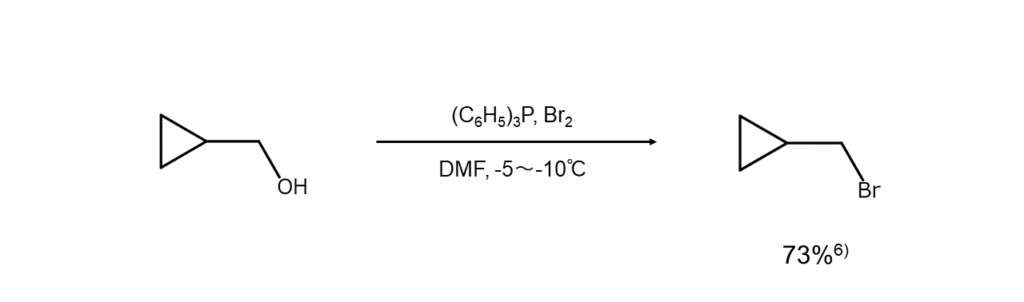
The examples below show bromoalkane synthesis from alcohols using PBr3.
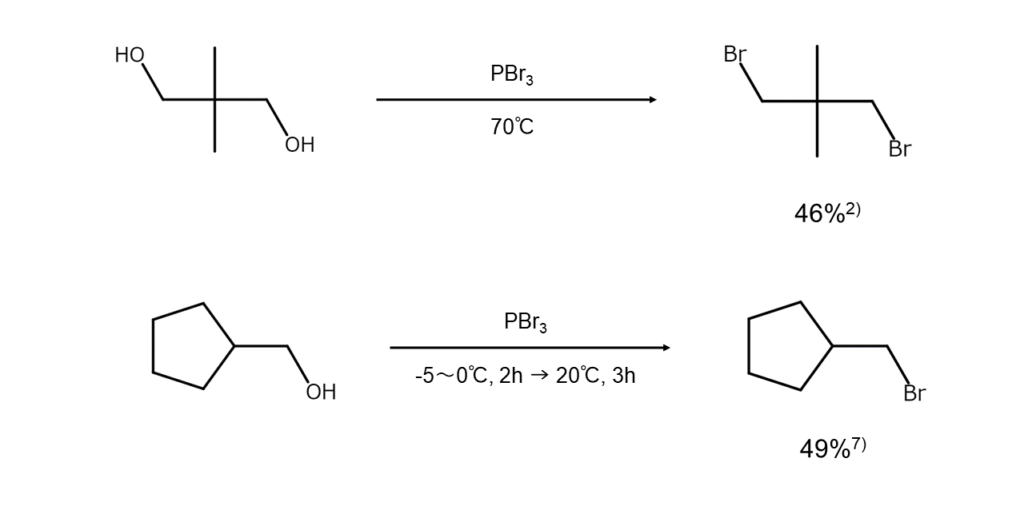
Effective for alcohols of various structures
As shown below, PBr3 can also convert alcohols with intramolecular double or triple bonds into bromides. However, as another approach for cases where hydrogen bromide additions or phosphite ester generation is unavoidable, alcohols can be changed into a tosylate (esters of p-toluenesulfonic acid) in order to treat the resulting product with an alkali bromide.
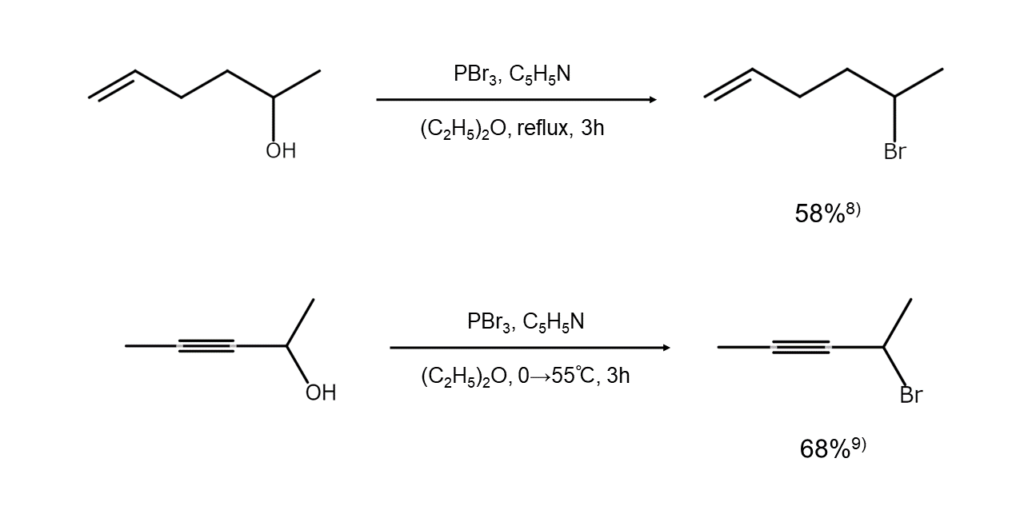
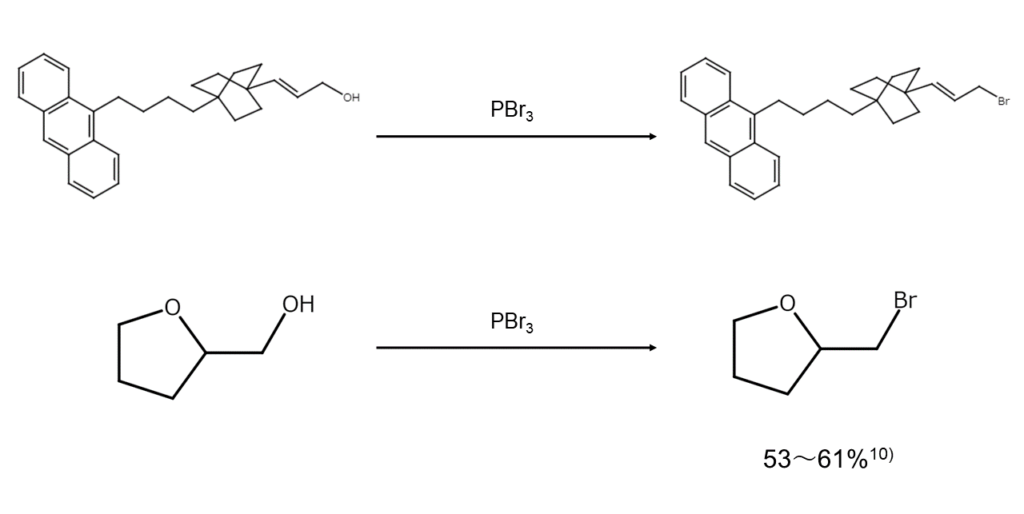
Any polyene structures or ether bonds present in the molecule can be left remaining while substituting the hydroxy groups with bromine atoms.
Furthermore, although not an alcohol, there are reports of great interest regarding reactions that convert O-silyl cyclopropanone acetals into alpha-bromoethers.
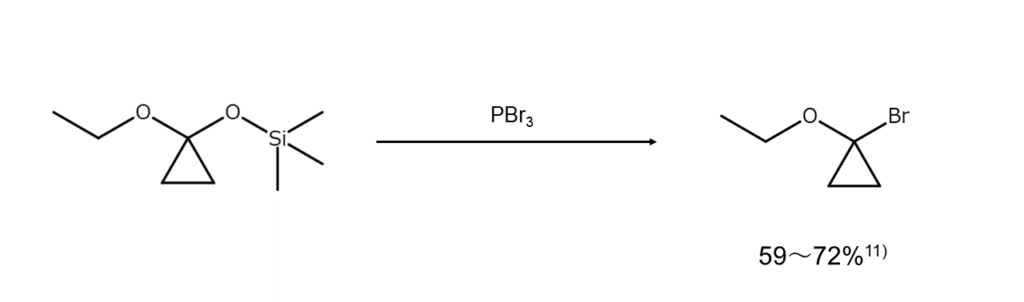
Caution required for PBr3 addition approaches and reaction temperatures
Reactions that synthesize bromoalkanes from alcohols using PBr3 are normally carried out through methods that add PBr3 dropwise into an alcohol. Care is needed as the opposite approach will easily cause runaway reactions. Attention should also be given to temperature control as there is a risk of generating dangerously toxic phosphine if the reaction is rapidly heated to more than 100°C.
Effective approaches for obtaining target products from post-reaction mixtures
Mixtures resulting from these reactions are generally processed using large volumes of water. However, excellent results can be achieved for acid-sensitive compounds by processing with methanol instead. In cases where phosphite esters occur in large amounts as byproducts, these can be prevented from contaminating the final product by using petroleum ether as an extraction solvent, or if the target product has a low boiling point, using steam distillation.
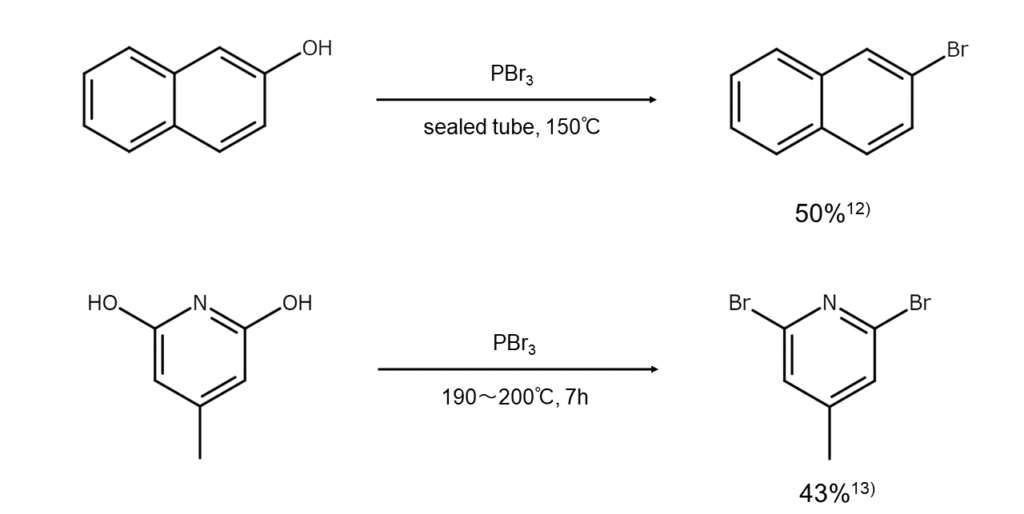
Reactions 2: Synthesizing bromoarenes from phenols
While lacking in generality, heating PBr3 for an extended period together with a compound such as 2-naphthol, 2-pyridinol/4-pyridinol, and 2-quinolinol/4-quinolinol will substitute the hydroxy groups with bromine atoms, converting them into the corresponding bromoarene or bromo(hetero)arene. These reactions do not produce strong yields as resinification readily occurs.
The following are examples of common reactions.
Column: Preventing polymer degradation – The power of phosphite esters
Earlier in the article, phosphite esters are presented as a “nuisance” of sorts in the form of byproducts when synthesizing bromoalkanes from alcohols. While phosphite esters can indeed present such challenges, word has it that they are rather helpful as antioxidants for preventing plastic degradation.
Plastics like polyethylene and polypropylene are staple materials in modern society owing to their numerous advantages, such as being inexpensive, light, easy to shape, and not subject to rust. However, continuous exposure to light or heat during or after manufacturing processes causes radical reactions that result in degradation. The addition of various stabilizers is, therefore, a standard practice as a way to prevent such degradation. One type of antioxidant used as a plastic stabilizer is none other than the phosphite ester.
Hydroperoxides (ROOH) occur in the degradation process of plastics (RH). Phosphite esters have a mechanism of action that takes ROOH and reduces it to ROH. The decomposition of ROOH, in turn, suppresses the occurrence of radicals, which then enables a reduction in degradation factors for plastics.14)
This exemplifies how a certain compound can be a nuisance as a byproduct in certain applications while also supporting our lifestyles as an antioxidant in others. It would seem that each compound has many different faces.
References
1) MANAC Inc. Research Lab., Suzuki, H. (ed.). Shuuso oyobi Youso Kagoubutsu no Yuuki Gousei: Shiyaku to Gouseihou [Organic Syntheses of Bromine & Iodine Compounds: Reagents & Synthesis Methods], Maruzen Publishing (2017).
2) Shortridge, R. W., Craig, R. A. et al. J. Am. Chem. Soc., 1948, 70, 946.
3) Goshorn, R. H., Boyd. T. Org. Synth. Coll. Vol. I, 36 (1941).
4) Dannley, R. L., Weschler, J. R. et al. J. Am. Chem. Soc., 1955, 77, 3643.
5) Kaslow, C. E., Marsh, M. M. J. Org. Chem., 1947, 12, 456.
6) Hrubiec, R. T., Smith, M. B. J. Org. Chem., 1984, 49, 431.
7) Noller, C. R., Adams, R. J. Am. Chem. Soc., 1926, 48, 1080.
8) Wood, jr. H. B., Horning, E. C. J. Am. Chem. Soc., 1953, 75, 5511.
9) Smith, L. I., Swenson, J. S. J. Am. Chem. Soc., 1957, 79, 2962.
10) Smith, L. H. Org. Synth. Coll. Vol. III, 793 (1955).
11) Miller, S. A., Gadwood, R. C. Org. Synth., 1989, 67, 210: Coll. Vol. VIII, 556 (1993).
12) Sah, P. P. T. Rec. trav. chim. Pays-Bas, 1940, 59, 1021.
13) Ames, D. E., Grey, T. F. J. Chem. Soc., 1955, 631.
14) Sumitomo Chemical Co., Ltd., Organic Synthesis Research Laboratory, Shinki Kousei Kakou Anteizai no Kaihatsu – Sumilizer GP– [Development of the Novel High Performance Processing Stabilizer – Sumilizer GP], (2002). <https://www.sumitomo-chem.co.jp/rd/report/files/docs/20020205_22s.pdf>













