A brominating agent that facilitates addition reactions with alkenes, overview and reaction mechanisms of N-bromoacetamide (NBA): N-bromo compounds (10): Discussion series on bromination/iodination reactions 10
Many different bromides are used as chemical intermediates in a wide range of areas, including in pharmaceuticals, agrochemicals, and semiconductor materials. Brominating agents such as N-bromo compounds are indispensable for producing these bromides.
When it comes to N-bromo compounds used as brominating agents, N-bromosuccinimide (NBS) or 1,3-dibromo-5,5-dimethylhydantoin (DBDMH) likely come to mind. However, there exists a particular brominating agent that exhibits unique reactivity that differs considerably from NBS and DBDMH. That certain brominating agent is known as N-bromoacetamide (NBA).
NBA is distinguished by its ability to easily bring about bromine addition reactions to alkene double bonds. NBA is better suited than NBS or DBDMH in cases where addition products, rather than substitution products, need to be obtained preferentially. When implemented properly, NBA can greatly lower the difficulty of certain reactions.
This article reviews the characteristics of NBA, handling precautions, and discusses bromination reactions that use NBA. Information in this article is provided as a reference to help achieve effective NBA applications.
■ What you can learn from this article ✔ NBA is a white to slightly yellow solid that is particularly used for addition reactions, such as the bromohydrination of alkenes and the oxidation of alcohols. ✔ Unlike NBS, NBA tends to prioritize addition reactions to alkenes over substitution reactions, with very little allylic bromination observed. ✔ Since NBA functions both as a brominating agent and an oxidizing agent, it is essential to select appropriate reaction conditions when oxidation is to be avoided. ■ Recommended Articles ・One of the most powerful brominating agents, overview and reaction mechanisms of dibromoisocyanuric acid (DBI): N-bromo compounds (11): Discussion series on bromination/iodination reactions 11 ・Benzylic brominations/aromatic ring brominations: DBDMH bromination reactions: N-bromo compounds (9): Discussion series on bromination/iodination reactions 9
contents
Describing N-bromoacetamide (NBA)
A brominating agent that easily facilitates addition reactions
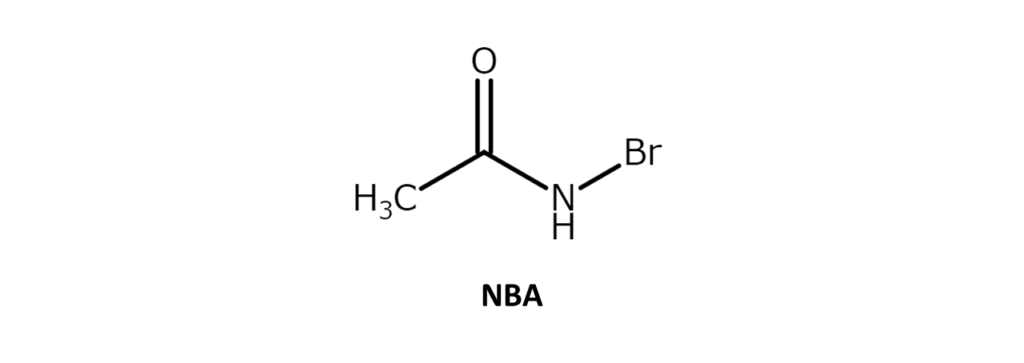
N-bromoacetamide (NBA) is a white to slightly yellow solid with a melting point of 102–105°C. While soluble in water and THF, the compound has poor solubility in hexane and ethers. NBA is used in various processes, such as the bromohydrination of alkenes and the oxidation of alcohols.
Unlike bromination reactions using the major brominating agent N-bromosuccinimide (NBS), bromination reactions using NBA are characterized by the ease of prioritizing addition reactions over substitution reactions. There is almost no observed tendency toward allylic bromination. Details regarding addition reactions are covered below.
NBA preparation method and precautions
NBA can easily be prepared through a method in which bromine is set to act on acetamide in an alkali hydroxide aqueous solution. However, even when stored in a cool, dark place, NBA steadily degrades over time, requiring the reagent to be used soon after preparation.
NBA handling precautions
There is a risk of NBA igniting or exploding if it comes into contact with reducing agents or is mixed with combustibles. Because NBA is a severe irritant for skin and mucous membranes, care must be taken to prevent such contact.
Bromination reactions with NBA: bromine addition to alkenes
Reaction details
Since the nitrogen atom in NBA is adjacent to an electron-withdrawing carbonyl group, the bromine atom in NBA is positively polarized and acts electrophilically on compounds rich in electrons. The bromine atom in NBA is added to alkene double bonds (electrophilic addition reaction) through these principles.
Bromination reactions with NBA advance by an ionic mechanism via a 3-membered ring bromonium ion intermediate with a positive bromine atom (structured with bromine bridging the two carbon atoms in the original double bond).
At first, bromonium ions form as a result of the NBA electrophilic attack. A nucleophilic agent then attacks the carbon atoms of the bromonium ions. As nucleophilic agents attack from the opposite side of the bromine atom, adducts with a 1,2 trans arrangement are usually obtained.
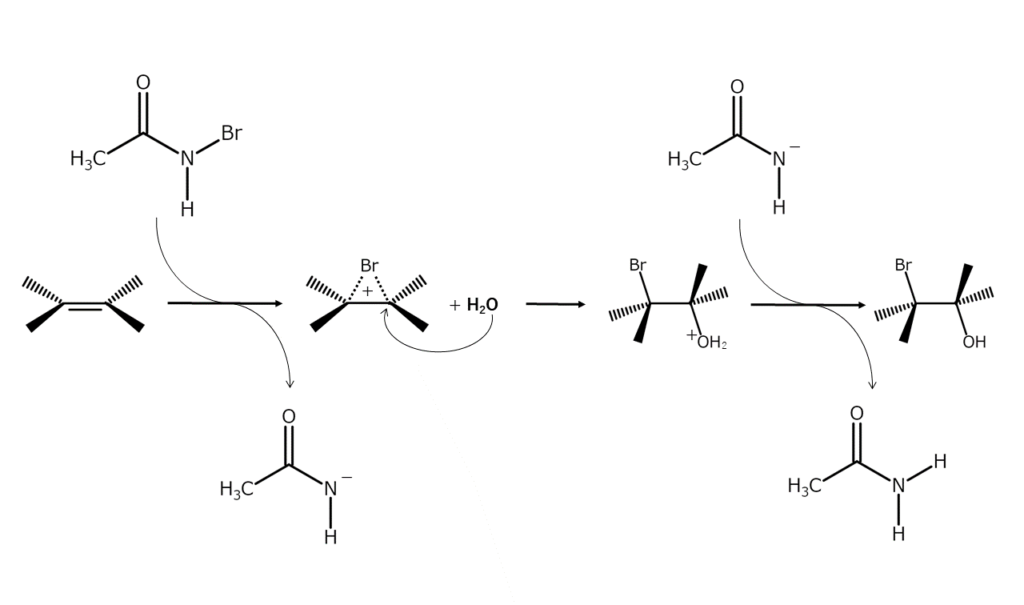
Obtaining various products with nucleophilic agent selection
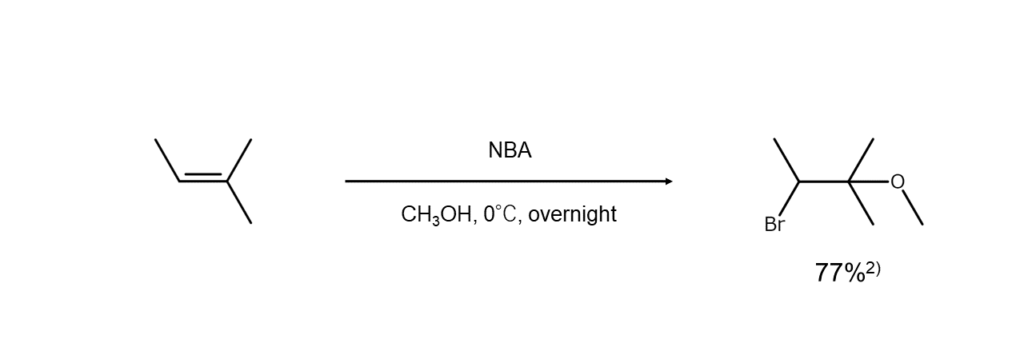
Depending on the nucleophilic agent used, different products can be obtained when using NBA for bromine addition reactions to alkenes. For example, when water is used as a nucleophilic agent, bromohydrins (bromoalcohols) are generated. Likewise, a bromohydrin ether is formed when the nucleophile is an alcohol. The following diagram illustrates a reaction using methanol as a nucleophilic agent to generate ether.
Additionally, esters are formed when carboxylic acid is used as a solvent, and if under the presence of a different type of halogen ion, a mixed dihaloalkane is yielded. Examples of these two reactions are shown below.
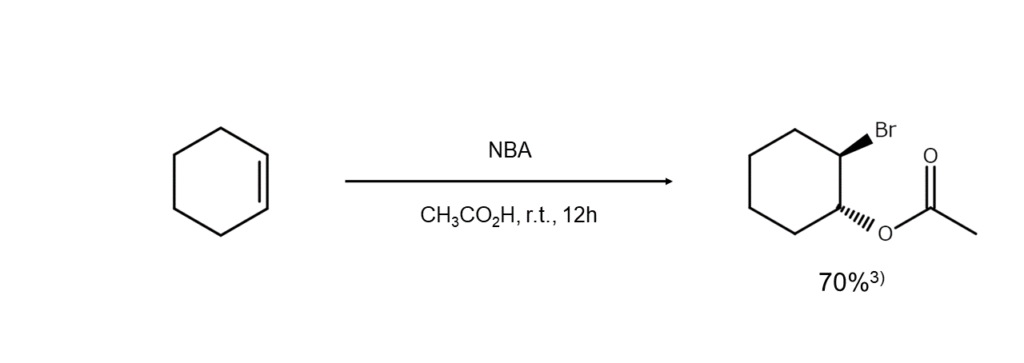
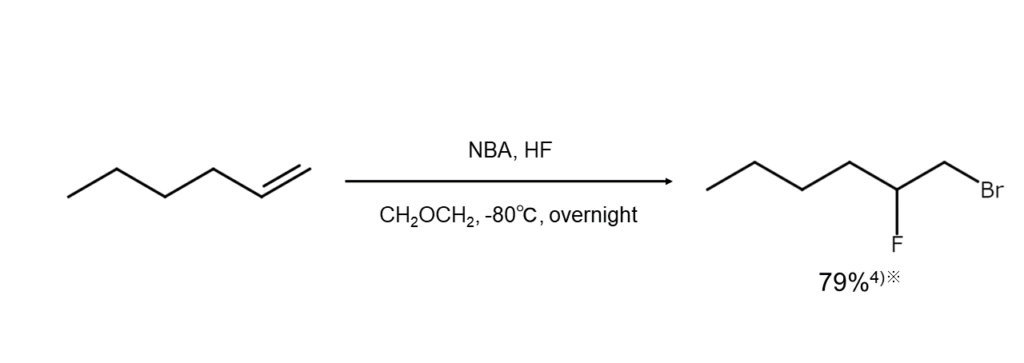
Using NBA for bromoamination
The bromoamination of alkenes can also be achieved with NBA. Researchers Chen and others5 have reported on the bromoamination reaction of β-nitrostyrene derivatives using tripotassium phosphate (K3PO4) as a catalyst. The following figure illustrates an example of this reaction.
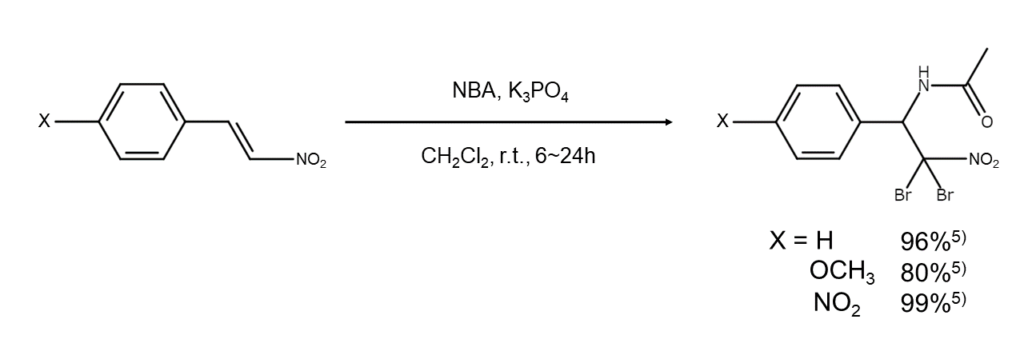
Using NBA as an oxidizing agent
N-bromo compounds are used not only as brominating agents but also as oxidizing agents. NBA is no exception.
For example, the oxidation of ketones, D-arabinose, and mannose using NBA has been reported.6,7 The oxidation of ethylene glycol and glycerol using ruthenium (III) catalysts is also being researched.8
When planning to use NBA as a brominating agent, it is important to consider reaction conditions in order to avoid unwanted oxidation reactions.
MANAC manufactures and sells NBS, a major N-bromo compound, as well as DBDMH. Please feel free to inquire.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing.
2) Winstein, S., Ingraham, L. L. J. Am. Chem. Soc., 1952, 74, 1160.
3) Schmidt, E., von Knilling, W. et al. Chem. Ber., 1926, 59, 1279.
4) Pattison, F. L. M., Peters, D. A. V. et al. Can. J. Chem., 1965, 43, 1689.
5) Chen, Z. G., Wang, Y. et al. J. Org. Chem., 2010, 75, 2085.
6) Singh, B., Srivastava, R. Tetrahedron, 1986, 42, 2749.
7) Singh, A. K., Srivastava, J., Rahmani, S. J. Mol. Catal. A Chem., 2007, 271, 151.
8) Singh, B., Singh, D., Singh, A. K. Int. J. Chem. Kinet., 1988, 20, 501.