
Bromoallene synthesis from propargv alcohol, ortho-selective bromination of aromatic rings: NBS bromination reactions (5): N-bromo compounds (7): Discussion series on bromination/iodination reactions 7
In this series, we discuss bromination and iodination reactions, specialties of MANAC. This article is the seventh of the series, continuing our coverage of various NBS bromination reactions.
This article covers two reactions: bromoallene synthesis from propargyl alcohol and ortho-selective bromination of aromatic rings. The former is a reaction that brominates compounds containing a propargyl alcohol structure, converting them into bromoallenes. The latter ortho-selectively brominates aromatic compounds under the presence of a metal complex catalyst. As this article reviews reaction mechanisms and example reactions, be sure to use this information as a reference in your research and experiments.
■ What you can learn from this article ✔ Reacting propargyl alcohol with NBS allows for the synthesis of bromoallenes. ✔ Allenes exhibit activity as enzyme inhibitors and antiviral agents in pharmaceuticals, and research is advancing in their development. ✔ By using metal complex catalysts, the ortho position of aromatic rings can be selectively brominated. ■ Recommended Articles ・ A fascinating brominating agent capable of minimizing costs and byproducts, describing 1,3-dibromo-5,5-dimethylhydantoin (DBDMH): N-bromo compounds (8): Discussion series on bromination/iodination reactions 8 ・ A-bromination of carbonyl groups, bromination of carboxylic acids and related compounds: NBS bromination reactions (4): N-bromo compounds (6): Discussion series on bromination/iodination reactions 6
contents
Describing N-bromosuccinimide (NBS)
A very commonly used, easy-to-handle brominating agent
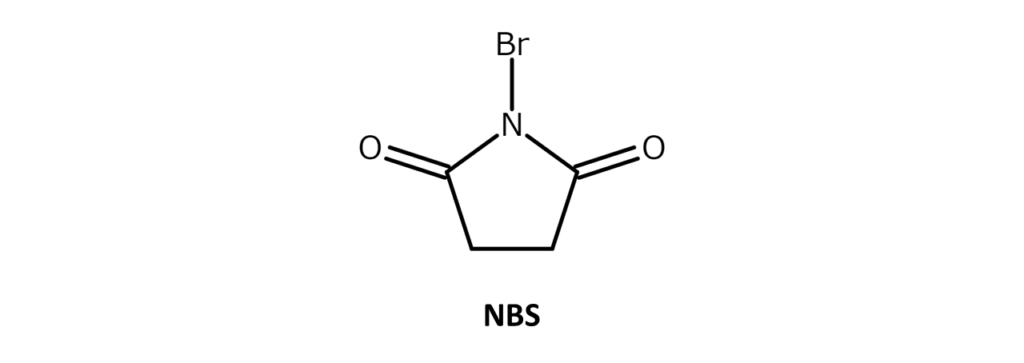
NBS is a white to slightly yellow crystalline powder with a weak bromine odor and a melting point of 173–176°C (decomposition). The compound is soluble in substances such as acetone, THF, DMF, acetonitrile, and DMSO, slightly soluble in water and acetic acid, and has poor solubility in hexane and carbon tetrachloride.
NBS powder is easier to handle than bromine (liquid), making it a common first-line brominating agent in organic syntheses. With its ability to undergo long-term storage in cool, dark, dry places, NBS is also relatively inexpensive.
This article covers information regarding the characteristics and precautions of NBS and gives an overview of NBS bromination reactions.
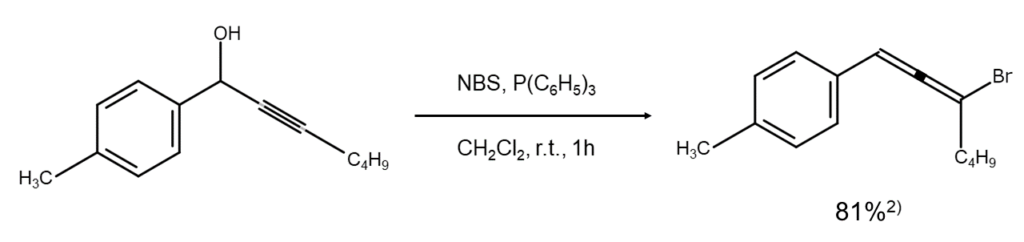
NBS bromination reactions: bromoallene synthesis from propargyl alcohol
Reaction details
Reacting propargyl alcohol with NBS in dichloromethane under the presence of triphenylphosphine allows for a medium to high yield of the corresponding bromoallene. The following figure illustrates an example of a reaction that converts a 1-(4-methylphenyl)-2-hepten-1-ol molecule containing a propargyl alcohol structure into a bromoallene.2)
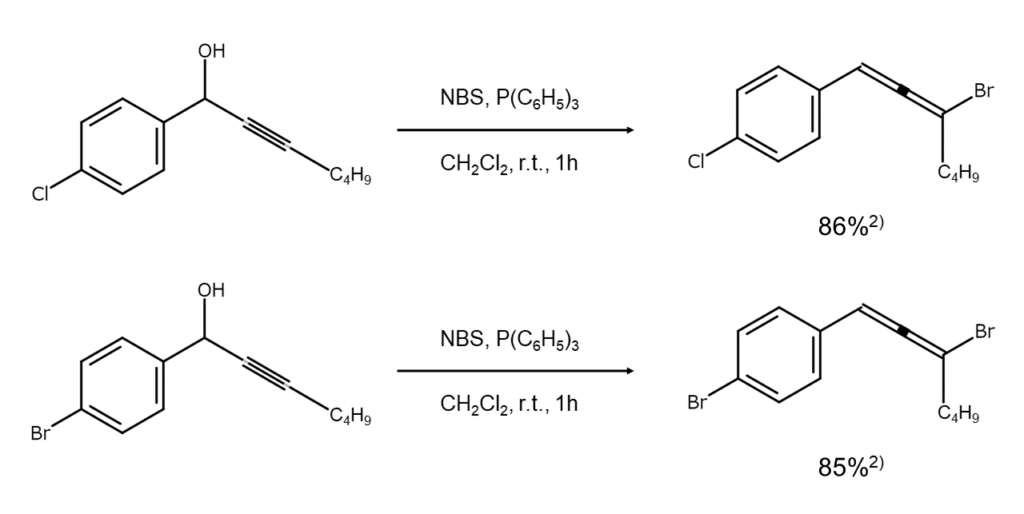
The above reaction process remains the same for reactants with a 4-chlorophenyl group or a 4-bromophenyl group instead of a 4-methylphenyl group.2)
[MANAC Tidbits] Application of allenes in pharmaceuticals
Compounds with two double bonds within the molecule (dienes) have the following classifications: Conjugated dienes, with two double bonds separated by one single bond; unconjugated dienes, with two double bonds separated by two or more single bonds; and allenes (cumulated dienes), with two adjacent double bonds. The product of the above reaction, bromoallenes, are compounds with a bromine atom bonded to the carbon atoms that comprise the double bond of an allene.
Naturally occurring allenes often exhibit biological and pharmacological activity, and research is advancing to develop compounds that introduce allene structures into skeletons classified as pharmacologically active (such as steroids, amino acids, and nucleosides). Certain compounds among these exhibit strong activity as enzyme inhibitors, cytotoxic agents, and antiviral agents.3) Developing efficient synthesis methods for allene derivatives would be ideal for further application of allene structures in the pharmaceuticals field.
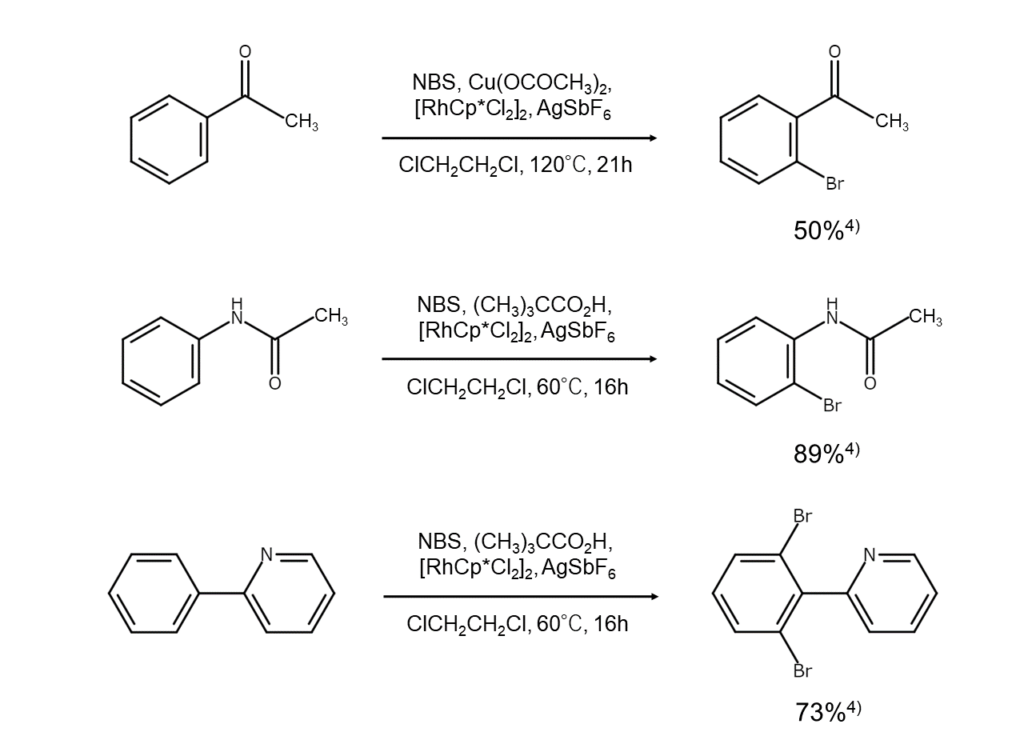
NBS bromination reactions: ortho-selective bromination of aromatic rings
Reaction details
This reaction features the use of metal complex catalysts.
An aromatic compound with a specific substituent, such as an amide group or pyridyl group, is reacted with NBS in the presence of a metal complex catalyst containing a platinum group metal (such as palladium or rhodium). Doing so results in a medium to high yield of ortho-selectively brominated compounds. This reaction is believed to progress via activation of the ortho C-H bond by the abovementioned metal complex catalyst.4)
The figures below illustrate example reactions of aromatic compound NBS ortho-bromination.
[MANAC Tidbits] Using catalysts to improve bromination regioselectivity
The type and position of previously introduced substituents affect the regioselectivity of aromatic electrophilic substitution reactions. When brominating aromatic rings containing multiple reaction points, it is crucial to prevent the bromination of non-targeted reaction points as much as possible. In particular, selective bromination of the ortho or para position of an aromatic ring with an ortho-para orientation substituent requires consideration of many different reaction conditions.
As is the case with the reactions reviewed in this article, the ortho position of aromatic rings containing specific substituents, such as amide groups and pyridyl groups, can be brominated at an elevated selectivity if certain types of catalysts are used. We recommend considering the ortho-selective bromination of aromatic rings with catalysts when major yield increases are needed for aromatic ring ortho-bromination or when an improved level of quality is demanded.
MANAC manufactures and sells NBS and DBDMH, two common N-bromo compounds. Please feel free to inquire.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing.
2) Du, X., Dai, Y. et al. Synth. Commun., 2009, 39, 3940.
3) Hoffmann-Röder, A., Krause, N. Angew. Chem. Int. Ed. 2004, 43, 1196.
4) Schröder, N., Wencel-Delord, J. et al. J. Am. Chem. Soc., 2012, 134, 8298.














