
MANAC’s three strengths in manufacturing active pharmaceutical ingredients and intermediates
MANAC’s facilities are equipped to manufacture active pharmaceutical ingredients (API) and intermediates. To date, MANAC has manufactured a large number of APIs and pharmaceutical intermediates. Just where do MANAC’s API manufacturing strengths lie? We sat down with MANAC business specialists to find out.
contents
Many results for investigational drugs and market launch pharmaceuticals
Over 60 years have passed since MANAC acquired its Drug Manufacturer License, and has been manufacturing pharmaceuticals ever since. At present, MANAC manufactures five APIs and six pharmaceutical intermediates (three of which are manufactured under GMP management). MANAC expanded its pharmaceutical manufacturing facilities about 10 years ago at its factory site in Fukuyama, Hiroshima, Japan, and has since continued manufacturing for investigational drugs and market launch pharmaceuticals.
A new drug was created by combining the intermediates manufactured by MANAC with the remaining required gynecological investigational drug intermediates produced by other companies.
MANAC also produces the hypervalent iodine compound DAIB, which is used as an oxidant in the synthesis process for active ingredients (APIs) that undergo various chemical reactions during production. While heavy-metal-based oxidants are generally used for oxidation reactions, DAIB is characterized as an oxidant by a wide range of applications owing to its low toxicity and environmentally friendly profile. The compound has garnered attention from many API manufacturers.
Mr. Go Tanaka (pictured at the beginning on the right), Division Manager of the Fine Chemical Division, commented on MANAC’s technologies. “Many API manufacturers have sought to leverage the technological capabilities we have amassed over the years and contracted with us for research and development.”
Strength 1: Final processes entirely conducted in a single clean room
MANAC’s pharmaceutical manufacturing facilities include the equipment listed in the figure below.
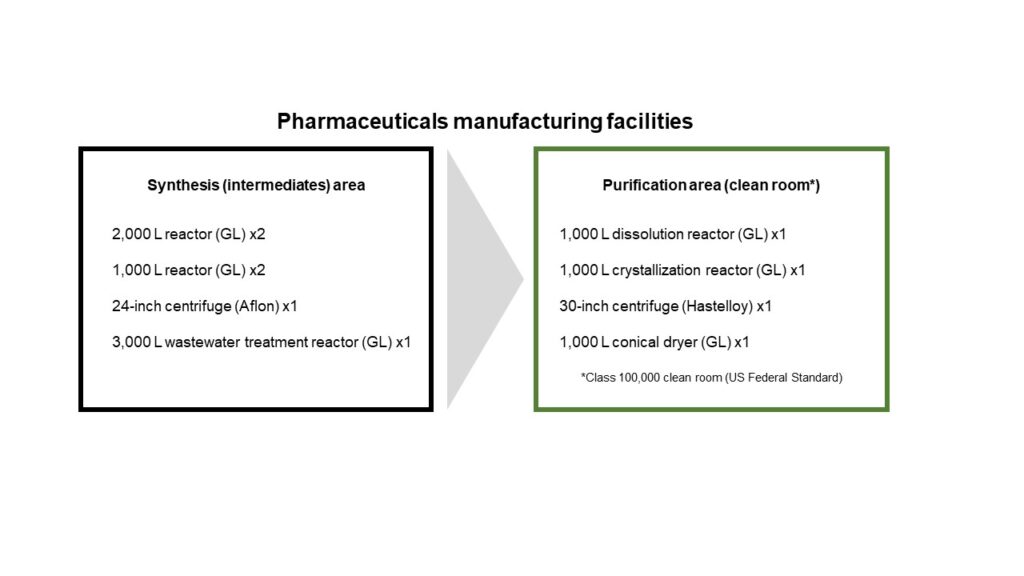
Mr. Akihiro Fukuta (pictured at the beginning on the left), Life Science Section Chief in the Fine Chemical Department, smiled as he described the equipment in MANAC’s pharmaceutical manufacturing facilities as “nothing outstanding by any standard,” but continued by explaining that “there is, indeed, a unique strength.”
This strength is manifested by having the crystallization reactor, centrifuge, and dryer in the same clean room.
When freshly synthesized, compounds are powders mixed in liquid. The mixture is placed in a centrifuge to separate the liquid and impurities in order to extract the compound powder at high purity.
Some clean rooms are sectioned into separate rooms by equipment and purpose. At MANAC, however, equipment for crystallization, centrifuge, and dryer processes are placed in the same clean room. Doing so makes it possible to realize a complete line of processes in an environment capable of preventing contamination and cross-contamination.

Strength 2: Bromination and iodination technologies
Another MANAC strength that Mr. Fukuta describes lies in its technologies. Specifically, “bromination/iodination technologies” and “bromine/iodine compound utilization technologies.”
MANAC’s core technologies are its bromination/iodination technologies, and the various bromine/iodine compounds that these technologies produce are widely used as pharmaceutical intermediates. MANAC has the ability to efficiently create higher-order compounds by fully leveraging the bromine/iodine compounds that it manufactures in-house.
This means MANAC can both provide bromine/iodine compounds and propose further streamlined processes by also conducting the procedures that follow compound acquisition.
Furthermore, through partnerships with domestic iodine manufacturers in Japan, MANAC promotes frameworks to collect used iodine, enabling them to propose processes with minimal environmental impacts. The importance of recycling iodine, a limited resource, in order to achieve stable and effective utilization is continuously growing.
Strength 3: The spirit of collaboration from early development phases
For many years, MANAC has been manufacturing and marketing halogen-containing compounds (halogen compounds), such as those that contain bromine or iodine.
MANAC also holds patents in the pharmaceutical field.
In one instance, by applying its technologies, MANAC’s recommendation for an alternative brominating agent was accepted by a manufacturer that initially requested a different brominating agent.

Mr. Tanaka explained. “Rather than simply producing compounds following the methods presented to us, we aim to propose synthesis methods that tap into MANAC’s wealth of technologies and offer greater advantages to clients. We strive to build relationships that enable us to work closely with clients from early development phases.”
By taking advantage of facilities that are not overly large in scale, MANAC can manufacture individual products to minimize the risk of contamination. The ability to deploy a four-team/three-shift production system under GMP management with four staff members for 24-hour operations is yet another strength at MANAC’s disposal.
Mr. Tanaka explained further. “We work to provide our technological capabilities to API manufacturers in order to achieve speedy development collaboration. With this approach, we aim to produce products that are even more ‘downstream’ (products more directly usable by consumers) than what we produce today.”
At the end of the interview, we asked Mr. Tanaka if he could share just one aspect of MANAC that surpasses the competition. Mr. Tanaka closed his eyes for a brief moment of thought and proceeded to answer.
“That would be our spirit of collaboration. Our dedication to working closely with client companies to assemble (develop) products.”
Moving forward, MANAC plans to further its focus, more than ever before, on research and development in the fields of APIs and fine chemicals. Be sure to keep MANAC’s technological capabilities in mind when considering embarking on compound development for new drugs or high-value-added products.


















