A tried-and-true synthesis method, describing iodination reactions with halogen exchange (1): Discussion series on bromination/iodination reactions 12
In this series, we discuss bromination and iodination reactions, specialties of MANAC. Up through the last issue, we detailed bromination reactions that use NBS, DBDMH, and other N-bromo compounds.
Bromides produced from brominating agents such as N-bromo compounds are used as intermediates in many fields, including pharmaceuticals and semiconductors. Equally important to these industries as bromides are iodides. Starting with this issue, we will look closely at iodination reactions, which are employed to create iodides.
We begin first with iodination reactions that utilize halogen exchange. Halogen exchange is a valuable iodide synthesis method that has long been used for various applications. Knowledge related to halogen exchange is essential to designing iodination reactions.
This article briefly overviews iodination reactions with halogen exchange and explains specific iodination reaction types and mechanisms. We aim to help readers further deepen their understanding of halogen exchange by providing this article as reference material.
■ What you can learn from this article ✔ Using halogen exchange broadens the range of compounds that can undergo iodination. ✔ The Finkelstein reaction, one type of halogen exchange reaction, has long been used for the synthesis of compounds like iodoalkanes. ✔ For halogen-iodine exchange, acetone is the ideal solvent, progressing more quickly than other solvents. ■ Recommended Articles ・ Iodoalkane synthesis: Iodination reactions with halogen exchange (2): Discussion series on bromination/iodination reactions 13
contents
Describing iodination reactions with halogen exchange
A method for converting chlorides and bromides into iodides
Halogen exchange is a method used to replace a halogen atom in a compound with a different halogen atom. This article discusses halogen exchange in terms of replacing a chlorine atom or a bromine atom with an iodine atom.
It may seem plausible to simply introduce iodine atoms directly into compounds without going through the trouble of using halogen exchange. Such methods do, indeed, exist. For example, using molecular iodine (I2) as an iodinating agent allows for the direct iodination of alkanes and alkenes. However, given its low reactivity, compounds that can be iodinated with molecular iodine are limited, which is why halogen exchange reactions are widely used.
Reaction principles
There are several types of halogen exchange reactions. Among these, one of the most commonly used types for iodination reactions is known as the Finkelstein reaction2.
In a Finkelstein reaction, the halogen atom of a halogen compound is replaced with a different halogen atom through a bimolecular nucleophilic substitution reaction (SN2 reaction). The Finkelstein reaction is a conventional method for synthesizing products, such as iodoalkanes, owing to the wide-ranging substrate applications it affords.
The reaction principles are as follows.
Excess alkali iodide is set to act on a chloride or a bromide in a polar solvent (such as acetone, 2-butanone, DMF, HMPA, etc.). The nucleophilicity of halide ions in a polar solvent increases in the following order: Cl– < Br– < I–. This results in the formation of iodides through an SN2 reaction. The reaction is reversible since I– serves as an excellent leaving group.
R-X + NaI ⇌ R-I + NaX X = Cl, Br
Among the compounds in the reaction formula above, NaI exhibits relatively high solubility in organic solvents. Conversely, NaCl and NaBr generally have poor solubility and easily precipitate as crystals from the reaction system. For example, NaI will dissolve at room temperature in acetone at 1.29 mol/L, while NaCl will only dissolve at 5.5 × 10-6 mol/L. This greatly shifts the equilibrium of the above reaction to the right, enabling iodoalkanes, the reaction product, to be obtained efficiently (60–100% yield).
However, it is known that using a tertiary alkyl halide as a substrate readily leads to a simultaneous formation of alkenes due to partial isomerization or dehydrohalogenation. A small amount of Fe2Cl6 or ZnCl2 present in the reaction system can suppress these side reactions to a certain extent.
Acetone as the perfect reaction solvent
Acetone is optimal as a solvent when carrying out a halogen-iodine exchange. When using acetone at room temperature, halogen exchange reactions between methyl bromide and NaI progress 520 times faster than in an aqueous solution and 370 times faster than in a methanol solution.
What types of iodination reactions use halogen exchange?
The reactions below are examples of iodination reactions that use halogen exchange. Each of these reactions will be discussed further in the coming articles.
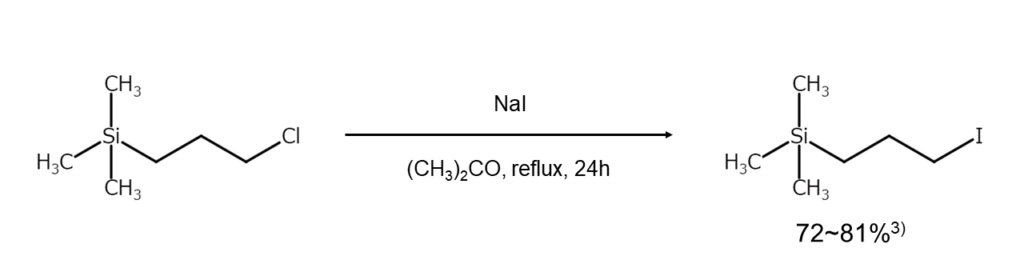
①Iodoalkane synthesis
This process utilizes the Finkelstein reaction to convert chloroalkane or bromoalkane into iodoalkane. As discussed above, these are generally reacted with an alkali iodide in a polar solvent.
②Acyl iodide synthesis
Similar to iodoalkane synthesis, the process for acyl iodide synthesis also entails the application of the Finkelstein reaction. However, unlike the process for iodoalkane synthesis, halogen exchange reactions can be conducted on tertiary acyl halides in acyl iodide synthesis without issue.

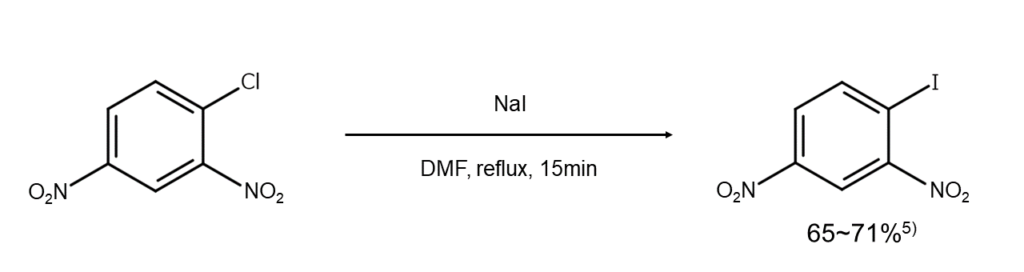
③Iodoarene synthesis
The Finkelstein reaction can synthesize iodoarenes when an electron-withdrawing group is present in the ortho position or para position of a halogen substituent. Grignard reagents or other reagents are used when an electron donating group is on a benzene ring since, in this case, nucleophilic substitution reactions do not readily occur.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing. (Japanese)
2) Finkelstein, H. Ber. 1910, 43, 1528.
3) Kuwajima, I., Urabe, H. Org. Synth. Coll. Vol. VIII, 486 (1993).
4) Schmidt, A. H., Russ, M. et al. Synthesis, 1981, 216.
5) Bunnett, J. F., Conner, R. M. J. Org. Chem., 1958, 23, 305; Org. Synth. Coll. Vol. V, 478 (1973).