
Bromination reactions with phosphorus bromides (bromine & phosphorus): Phosphorus bromides (2): Discussion series on bromination/iodination reactions 40
In this series, we discuss bromination and iodination reactions, specialties of MANAC. In the previous article, we launched our exploration of bromination reactions with phosphorus bromides.
Our focus in this article is on bromination methods that use bromine and phosphorus. These methods feature a certain compound that is generated from bromine and phosphorus and functions as a brominating reagent. What might this “certain compound” be, you ask? And just what advantages are there to generating this “certain compound” within the reaction system?
To find out this and more, be sure to read this article to the very end.
contents
Bromination reactions with phosphorus bromides: Reactions with bromine & phosphorus
Reactions 1: Synthesizing bromoalkanes from alcohols
Utilizing the PBr3 generated from bromine and phosphorus
As we touched upon in the previous article, PBr3 is often used in the laboratory when converting an alcohol into a bromoalkane. However, since large-scale bromoalkane synthesis requires the purchase of large quantities of PBr3, the compound is not very economical for this purpose.
The solution to this dilemma lies in the focus of this article: methods that use bromine and phosphorus. In these methods, bromine is reacted with the phosphorus in the substrate alcohol, generating PBr3, which is then used in situ to carry out bromination. As this eliminates the need to purchase expensive PBr3, these methods allow for such reactions at a lower cost.
Adding bromine dropwise to alcohol and red phosphorus mixtures
Yellow phosphorus dissolves in organic solvents and has excellent reactivity. However, it is also highly toxic and exhibits a high level of pyrophoricity. In turn, red phosphorus, with its non-toxic, stable properties, is generally used by suspending it in the substrate alcohol.
The reaction mixture of alcohol and red phosphorus is vigorously stirred while adding in bromine dropwise at a rate that generates enough reaction heat to maintain a gentle boil. The appropriate amount to use is roughly 2 mol of bromine per 1 gram atom of phosphorus. Caution is required when using vacuum-dried red phosphorus as the reaction may runaway following an induction period.
Here are some examples of bromoalkane synthesis from alcohols using bromine and phosphorus.
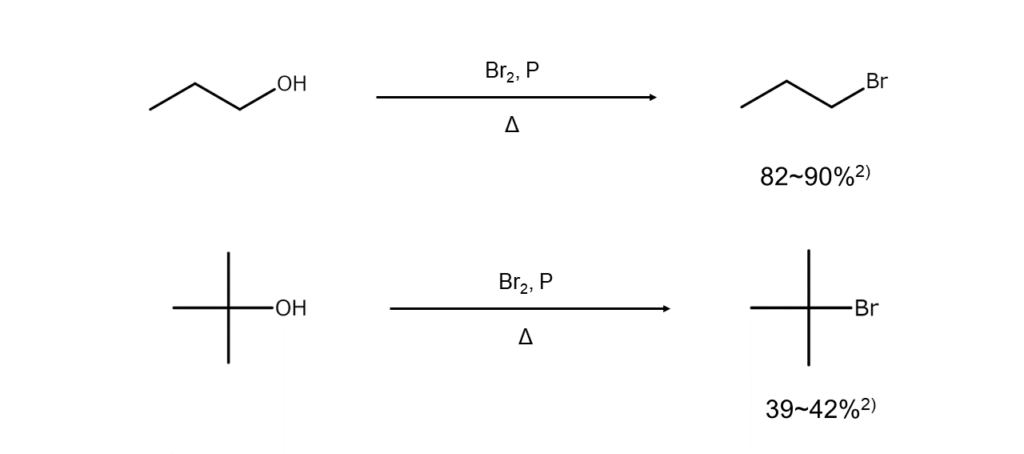
Reactions 2: Alpha-bromination of carboxylic acids (Hell-Volhard-Zelinsky reactions)
Brominating carboxylic acids using red phosphorus and bromine
Using red phosphorus and bromine allows for the bromination of not only alcohols but also carboxylic acids. One such example is the Hell-Volhard-Zelinsky reaction, a long-established carboxylic acid bromination method. The following illustrates specific reaction mechanisms.
Formula 1: RCH2C(=O)OH + PBr3 → RCH2C(=O)Br + HBr
Formula 2: RCH2C(=O)Br + Br2 → RCHBrC(=O)Br + HBr
Formula 3: RCHBrC(=O)Br + H2O → RCHBrC(=O)OH
Formula 4: RCHBrC(=O)Br + R’OH → RCHBrC(=O)OR’
First, the red phosphorus and bromine reaction generates PBr3 in situ. The PBr3 changes the carboxylic acid into an acyl bromide (Formula 1 above). Substituting red phosphorus in this step with other phosphorus(III) halides or thionyl chloride has been found to give similar results.
Next, the acyl bromide is tautomerized to an enol, which reacts with bromine to generate the acyl bromide of an alpha-bromocarboxylic acid (Formula 2 above). Processing this with water will yield an alpha-bromocarboxylic acid (Formula 3 above), while processing with an alcohol yields an alpha-bromoester (Formula 4 above).
Here are a few synthesis examples of alpha-bromocarboxylic acids and their esters using bromine and red phosphorus.
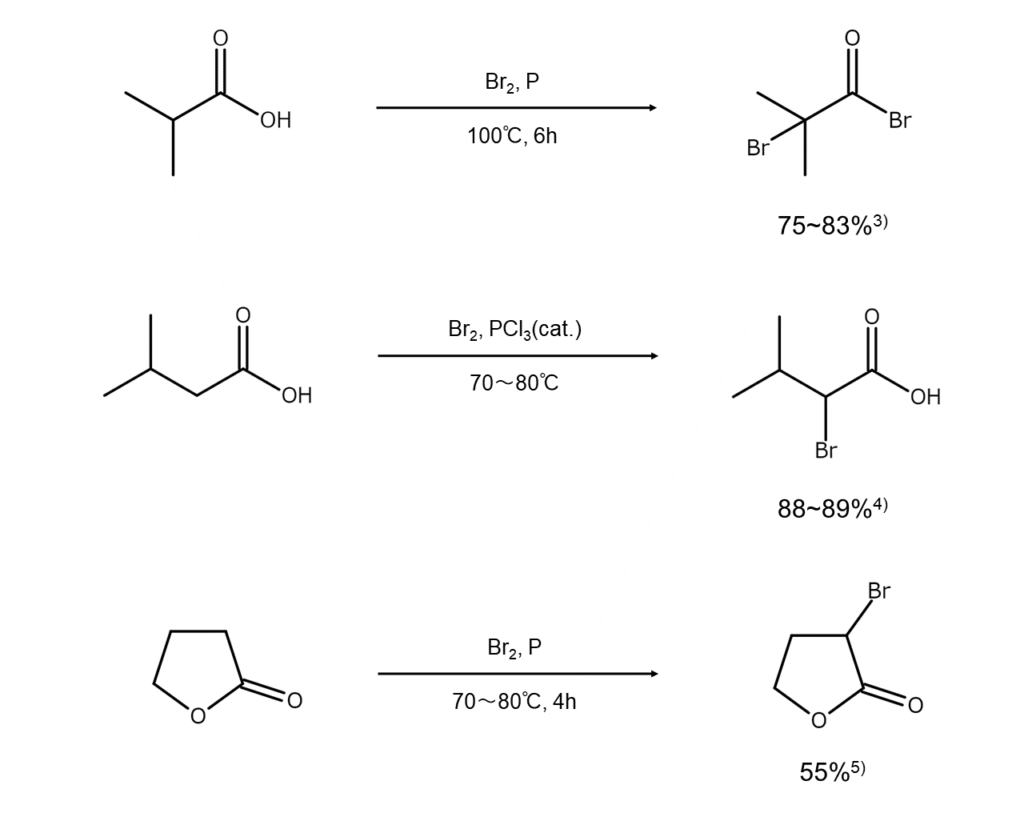
Not usable for unsaturated carboxylic acids
While the Hell-Volhard-Zelinsky reaction is quite useful, as illustrated above, it does not effectively yield alpha-bromocarboxylic acids when used on unsaturated carboxylic acids. In such applications, bromine additions to olefin bonds occur preferentially. This exemplifies why it is important to be attentive to substrate structures when using a Hell-Volhard-Zelinsky reaction.
Column: More options for synthesizing bromoalkanes from alcohols – Taking a glance at brominations using alkali bromides
So far in this series, we have taken a tour of the various methods used to synthesize bromoalkanes from alcohols. For example, methods that use hydrogen bromide, methods that use PBr3, as covered in the previous issue, and methods that use phosphorus and bromine, as reviewed in this article.
We have covered a lot of ground, but many other methods remain for synthesizing bromoalkanes from alcohols. One such example is an approach that changes an alcohol into a sulfonic acid ester to react with an alkali bromide. Since this approach can be taken in nearly neutral conditions, it is perfect for alcohols with allyl structures prone to acid-driven isomerization, as well as alcohols that contain acid-sensitive protecting groups.
Commonly used reaction intermediates include the ethers (tosylates) of p-toluenesulfonic acid (“TsOH”) (4-CH3C6H4SO3H) and the ethers (mesylates) of methanesulfonic acid (“MsOH”) (CH3SO3H). Reacting p-toluenesulfonyl chloride or methylsulfonyl chloride with the target alcohol in the presence of pyridine will readily synthesize these intermediates, which are generally used without isolation or any other further processing.
In the reaction example below, the OH groups in the alcohol are first converted into TsO groups, which are then reacted with lithium bromide (LiBr) to be replaced by bromine (Br).
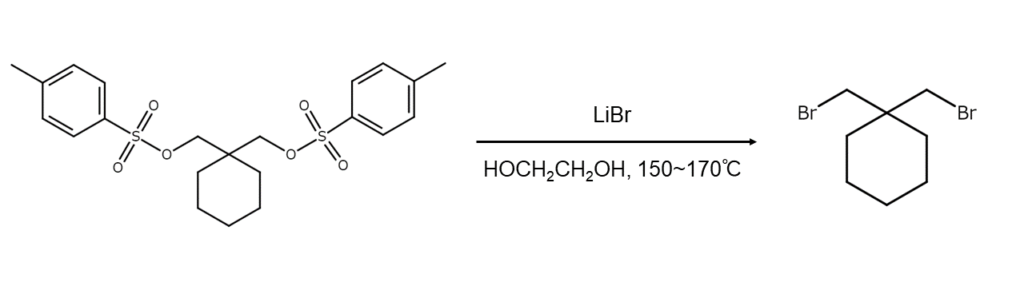
There are certainly many options available for synthesizing bromoalkanes from alcohols. We encourage you to review each reaction in detail so that you may have the best methods at your disposal.
References
1) MANAC Inc. Research Lab., Suzuki, H. (ed.). Shuuso oyobi Youso Kagoubutsu no Yuuki Gousei: Shiyaku to Gouseihou [Organic Syntheses of Bromine & Iodine Compounds: Reagents & Synthesis Methods], Maruzen Publishing (2017).
2) Goshorn, R. H., Boyd. T, Org. Synth. Coll. Vol. I, 36 (1941).
3)Smith, C. W., Norton, D. G. Org. Synth. Coll. Vol. IV, 348 (1963).
4) Marvel, C. S. Org. Synth. Coll. Vol. III, 848 (1955).
5)Price, C. C., Judge, J. M. Org. Synth. Coll. Vol. V, 255 (1973).













