
Bromination reactions with hydrogen bromide: Hydrogen bromide (1): Discussion series on bromination/iodination reactions 34
In this series, we discuss bromination and iodination reactions, specialties of MANAC. Here, we begin our exploration of bromination reactions that use hydrogen bromide.
Hydrogen bromide and its aqueous solution, hydrobromic acid, are well-known reagents for bromination reactions. These reagents are characterized by the useful ability to synthesize different reaction products from the same substrates depending on the reaction conditions. Without a doubt, these are essential reagents to cover when learning about bromination reactions.
This article begins with an overview of bromination reactions that use hydrogen bromide. We then discuss details on the properties and precautions of hydrogen bromide and hydrobromic acid. Be sure to use this information as a reference for experiments.
contents
Bromination reactions with hydrogen bromide
Common bromination reactions conducted under acidic conditions
Hydrogen bromide (HBr) is a colorless, irritating gas commonly used as a reagent to perform bromination reactions under acidic conditions.
▽ Conversions to bromoalkanes or bromoalkenes, such as for alkenes, alkynes, or alcohols
▽ Brominative cleavage of ethers and lactones
▽ Aromatic ring bromomethylation
▽ Conversions of nitriles into bromoimines
These four are examples of the various applications of hydrogen bromide.
In some cases, when hydrogen bromide is combined with an oxidant, the resulting bromine is used in situ as a brominating agent.
The aqueous solution of hydrogen bromide is known as hydrobromic acid. While hydrogen bromide easily dissolves in various polar and non-polar solvents, it is generally used as hydrobromic acid in the laboratory.
The mechanisms involved regarding hydrogen bromide in bromination reactions are varied.
For example, the bromine ion (Br–) is involved when bromination takes place in a polar solvent, and the bromine atom (Br⋅) is involved when the reaction takes place in a non-polar solvent (if light or a radical reaction initiator is present). A major advantage of such variation is the ability to obtain different reaction products with the same substrate and reagent combinations just by adjusting the reaction conditions.
List of bromination reactions with hydrogen bromide
Upcoming articles in this series will discuss the bromination reactions below.
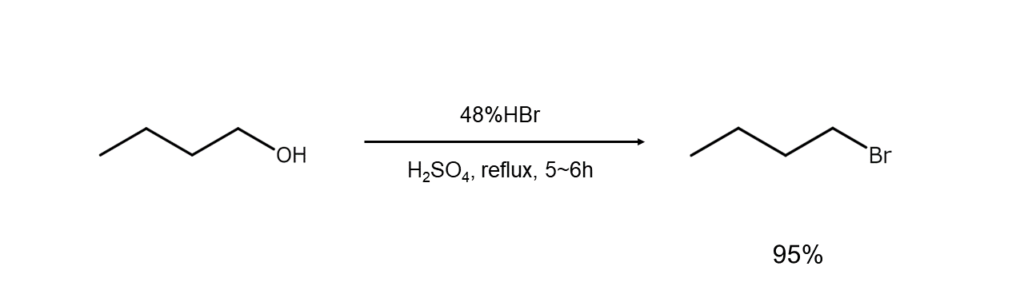
① Synthesis of bromoalkanes from alcohols
Synthesizing bromoalkanes from alcohols is a standard method. While primary and secondary alcohols require gentle heating with hydrobromic acid, reactions will occur for tertiary alcohols simply by combining them with the reagent and stirring at room temperature.
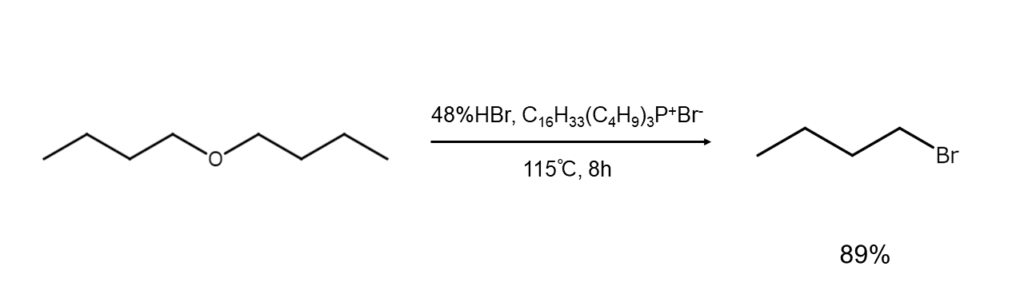
② Synthesis of bromoalkanes through ether- or lactone-cleaving
Ethers are cleaved through the action of hydrogen bromide or hydrobromic acid, yielding bromoalkanes and alcohols. The reaction mechanism differs depending on the substrates.
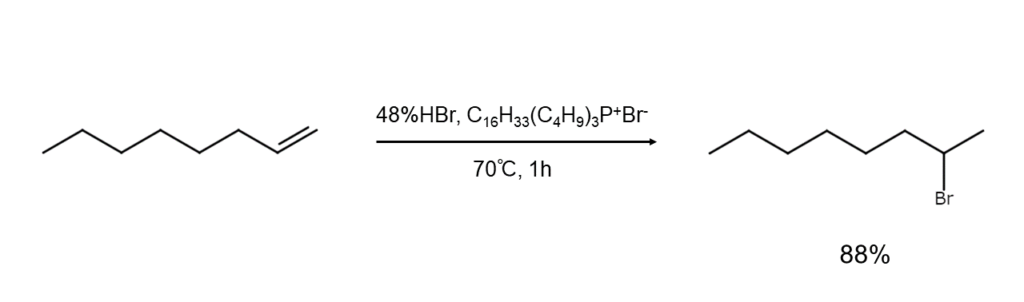
③ Additions to alkenes
Hydrogen bromide is easily added to alkenes at room temperature, generating bromoalkane. A Markovnikov addition will occur in a polar solvent, while an anti-Markovnikov addition will occur in a non-polar solvent.
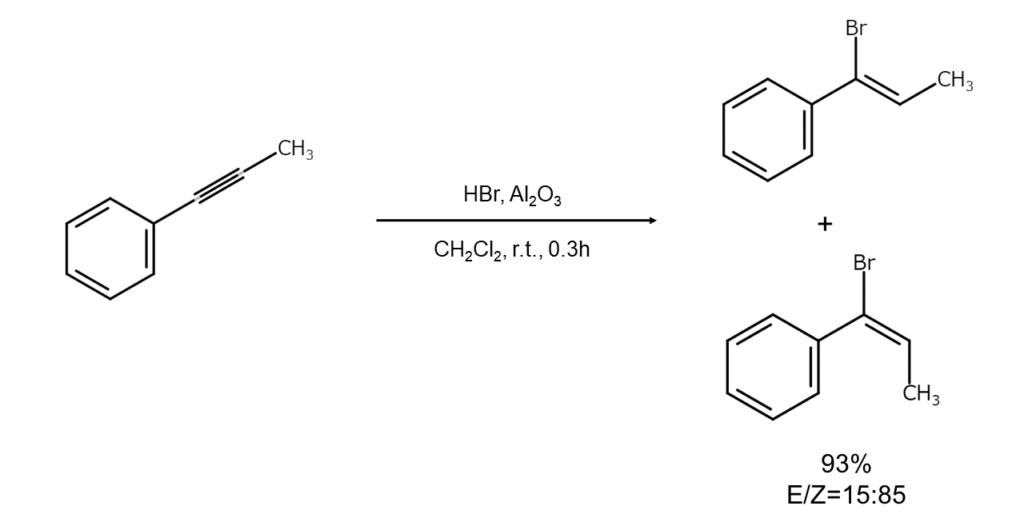
④ Additions to alkynes
Because hydrogen bromide addition reactions for alkynes are usually slow, catalysts such as CuBr are generally utilized. The differences in the direction of addition between reactions using polar solvents and those using non-polar solvents are not as apparent as they are for alkenes.
⑤ Bromomethylation
Using hydrogen bromide with paraformaldehyde will lead to the O-bromomethylation of an alcohol and produce high yields of bromomethyl ether. Further, reacting an aromatic hydrocarbon with hydrogen bromide and formaldehyde will yield the corresponding benzyl bromide.
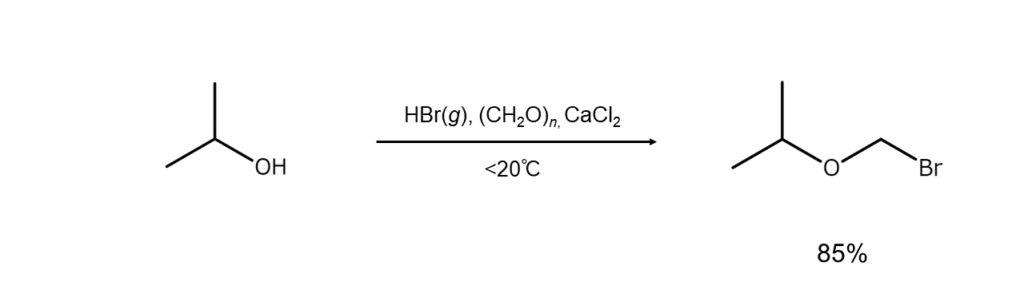
⑥ Bromination combining hydrogen bromide with an oxidant
This method combines hydrogen bromide with an oxidant to generate bromine, which is used in situ as a brominating agent. Reactions can be conducted while keeping bromine at exceptionally low concentration levels.
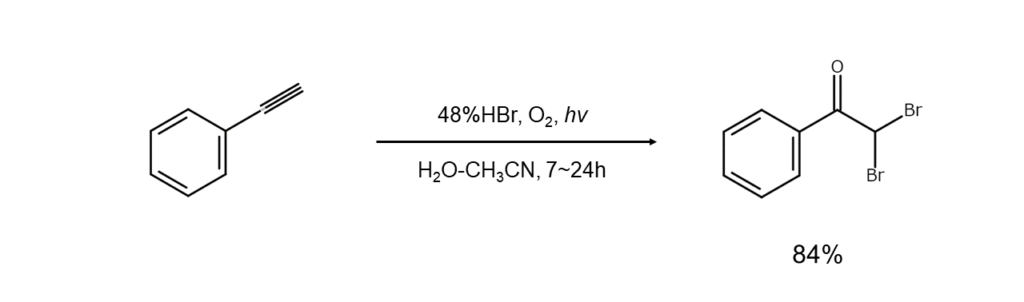
⑦ Other synthesis methods with hydrogen bromide
We will provide explanations of bromine atom transfer reactions and other reactions centered on activated aromatic bromine compounds.
Characteristics and Precautions regarding hydrogen bromide and hydrobromic acid
This final section reviews the compound characteristics and precautions of hydrogen bromide and hydrobromic acid. Please confirm these points before using these reagents in any experiments.
Hydrogen bromide
Hydrogen bromide (molecular weight: 80.912 g/mol) is a colorless, irritating gas that is fire-resistant and forms white fumes in humid air. While the compound is exceptionally stable, at high temperatures, small amounts will break down into bromine and hydrogen (0.0035% at 329°C and 1.08% at 1,222°C). It readily dissolves in water, acetic acid, alcohol, ether, liquid SO2, and other oxygen-containing solvents.
Even when diluted, hydrogen bromide will severely irritate the upper respiratory tract and mucous membranes, and so exposure must be avoided. If inhaled, the compound will cause violent coughing, a burning sensation in the throat, chest pain, and inflammation accompanied by submucosal necrosis. The permissible exposure limit of hydrogen bromide in the US is 3 ppm (up to 9.9 mg/m3) and 2 ppm in Germany (6.7 mg/m3).
Hydrobromic acid
Hydrobromic acid is the aqueous solution of hydrogen bromide and is a strong acid with a pKa less than -7. The aqueous solution is generally marketed at 47.63% since it forms an azeotropic mixture (boiling point: 124.3°C) at this concentration.
Pure hydrobromic acid is a colorless, fuming liquid. However, hydrobromic acid slowly oxidizes during storage, causing the release of bromine and a gradual yellowing. Light exposure promotes yellowing, so hydrobromic acid should be stored in a dark-colored glass container.
Column: The role of hydrogen bromide in hydrogen production processes
Amid the global movement to “become carbon neutral by 2050,”2) hydrogen is gaining attention as a new fuel to replace fossil fuels. Since hydrogen can be manufactured from various resources and does not emit carbon dioxide when used (burned as fuel or charged in a fuel cell), it is believed to hold great potential to contribute toward realizing carbon neutrality.3)4)
One of the known technologies for producing hydrogen is thermochemical water splitting. It is here that the hero of this article, hydrogen bromide, makes its entrance.
Thermochemical water splitting is a method that uses relatively lower temperatures to divide the water splitting reaction (H2O → H2 + 1/2O2) into multiple chemical reaction steps. Water splitting via direct heat energy alone requires high temperatures of around 4,000°C. However, using thermochemical splitting allows the process to take place at temperatures between 400°C and 1,500°C.5)-7)
There are many different methods for thermochemical water splitting. The process in which hydrogen bromide plays a role is a method known as the sulfur-bromine process, also known as the Ispra Mark 13 process.5)6) As shown below, this method reacts water with bromine and sulfur oxide to synthesize hydrogen bromide and sulfuric acid. Subjecting the hydrogen bromide and sulfuric acid to thermal decomposition produces hydrogen and oxygen, respectively.
Br2 + SO2 + 2H2O → 2HBr + H2SO4
2HBr → H2 + Br2
H2SO4 → H2O + SO2 + 1/2O2
Since sunlight can be used as the heat source in thermochemical water splitting, there are expectations from various fields for this process as a carbon-free hydrogen production method.8) Research and development will undoubtedly continue advancing toward realizing a hydrogen-based society.
MANAC is a global leader in bromination and iodination reactions. Please inquire with us about any bromination or iodination needs.
References
1) MANAC Inc. Research Lab., Suzuki, H. (ed.). Shuuso Oyobi Youso Kagoubutsu no Yuuki Gousei: Shiyaku to Gouseihou [Organic Syntheses of Bromine & Iodine Compounds: Reagents & Synthesis Methods], Maruzen Publishing (2017).
2) Agency for Natural Resources and Energy, Japan’s Ministry of Economy, Trade and Industry, “Reiwa 2-Nendo Enerugii ni Kansuru Nenji Houkoku, Dai-1-Bu Enerugii wo Meguru Joukyou to Omona Taisaku, Dai-2-Shou, 2050-Nen Kaabon Nyuutoraru Jitsugen ni Muketa Kadai to Torikumi, Dai-2-Setsu Shogaikoku ni Okeru Datsutansoka no Doukou” [Energy White Paper 2021, Part 1: Current Energy Situation and Key Measures, Chapter 2: Efforts and challenges toward carbon neutrality by 2050, Section 2: International trends toward decarbonization],
https://www.enecho.meti.go.jp/about/whitepaper/2021/html/1-2-2.html
3) Agency for Natural Resources and Energy, Japan’s Ministry of Economy, Trade and Industry, “Kaabon Nyuutoraru Jitsugen ni Muketa Kagi to Naru ‘Suiso’” [The Key to Realizing Carbon Neutrality – Hydrogen],
https://www.enecho.meti.go.jp/category/saving_and_new/advanced_systems/hydrogen_society/
4) Japan Patent Office, “Reiwa 4-Nendo Tokkyo Shutsugan Gijutsu Doukou Chousa Houkokusho, Kaabon Nyuutoraru ni Muketa Suiso/Anmonia Gijutsu” [FY2022 Survey Report on Technology Trends in Patent Applications – Hydrogen/Ammonia Technologies Toward Carbon Neutrality],
https://www.jpo.go.jp/resources/report/gidou-houkoku/tokkyo/document/index/2022_05.pdf
5) Journal of the Surface Science Society of Japan, Vol. 36, No. 2, pp. 80-85, 2015 Special Issue, “Suiso no Tsukurikata” [Hydrogen Production],
https://www.jstage.jst.go.jp/article/jsssj/36/2/36_80/_pdf/-char/ja
6) Matsumoto, H. (Kyushu University), “Suiso Seizou Shisutemu (Dai-7-Kai) Netsu Kagaku Suiso Seizou” [Hydrogen Production Systems (7th Ed.) Thermochemical Hydrogen Production],
https://i2cner.kyushu-u.ac.jp/~matsumoto/wp01/wp-content/uploads/2018/12/IM820_HydrogenProductionSystem_7_2018.pdf
7) Miyaoka, H., Kojima, Y., Ichikawa, T. (Hiroshima University), “Teion Netsukagaku Suibunkai ni Yoru Suiso Seisei Gijutsu” [Hydrogen Purification Technologies Via Low-temperature Thermochemical Water Splitting],
https://www.hiroshima-u.ac.jp/system/files/42424/kaiken-siryou2.pdf
8) Japan Atomic Energy Agency, HTGR Hydrogen and Heat Application Center, “R&D on Thermochemical IS Process”,
https://www.jaea.go.jp/04/o-arai/nhc/jp/faq/is_process.html












