
Describing iodosyl and iminoiodo compounds: Hypervalent organoiodine compounds (4): Discussion series on bromination/iodination reactions 32
Continuing from the previous article, we will discuss hypervalent organoiodine compounds.
Hypervalent organoiodine compounds have many applications, including as oxidants and dehydrogenating agents. Since they can be used as alternative reagents in place of toxic elements, hypervalent organoiodine compounds are exceedingly valuable for researchers seeking to conduct green chemistry research.
This article discusses iodosyl compounds and iminoiodo compounds, both different types of hypervalent organic compounds. Be sure to read the entire article as we also review one of the latest research papers.
contents
Hypervalent organoiodine compounds
Organic compounds that contain hypervalent iodine
A hypervalent organoiodine compound is just that — an organoiodine compound that is hypervalent.
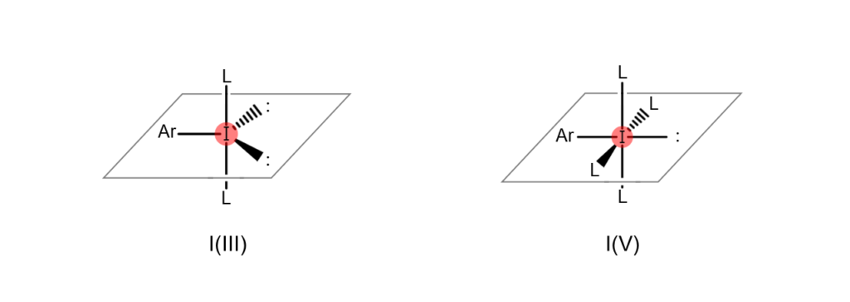
Atoms are “hypervalent” when the most outer electron shell (the part of the atom related to bonds with other atoms), which sits furthest from the nucleus, formally contains more than eight electrons. This state enables the atom to form a higher number of bonds with other atoms. For example, iodine atoms gain the ability to bond with multiple atoms at once when they become hypervalent, as shown in the figures below (the iodine atom on the left is trivalent, while the iodine atom on the right is pentavalent). When an organic compound contains hypervalent iodine, it is known as a “hypervalent organoiodine compound.”
Introducing hypervalent organoiodine compounds: Iodosyl compounds
Iodosylbenzene, a well-known iodosyl compound
Iodosyl compounds are compounds that contain an iodosyl group (IO). Here, let’s take a close look at iodosylbenzene (shown in the figure below), a well-known iodosyl compound.
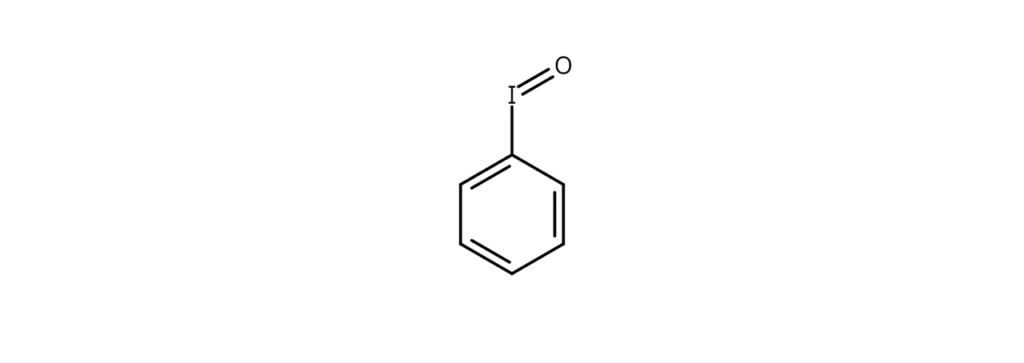
Iodosylbenzene (also known as iodosobenzene) is an amorphous white powder. Although the compound explosively decomposes near its melting point of 210°C when heated, it can be stored over the long term in cool, dark conditions.
Iodosylbenzene can easily be obtained by subjecting (dichloroiodo)benzene or (diacetoxyiodo)benzene to hydrolysis under alkaline conditions, and has long been used as a mild oxidant or arylating agent. Furthermore, since iodosylbenzene is also an important raw material for synthesizing hypervalent organoiodine compounds, it is relatively inexpensive on the market.
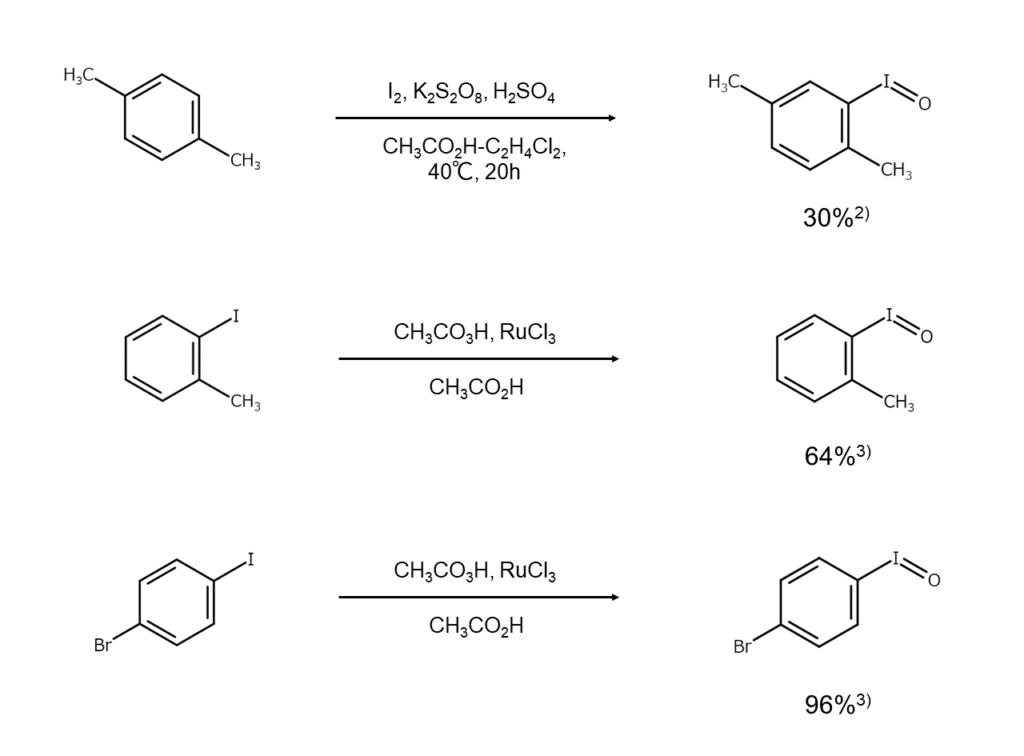
Currently, many iodosylarenes other than iodosylbenzene are synthesized. Here are a few reaction examples.
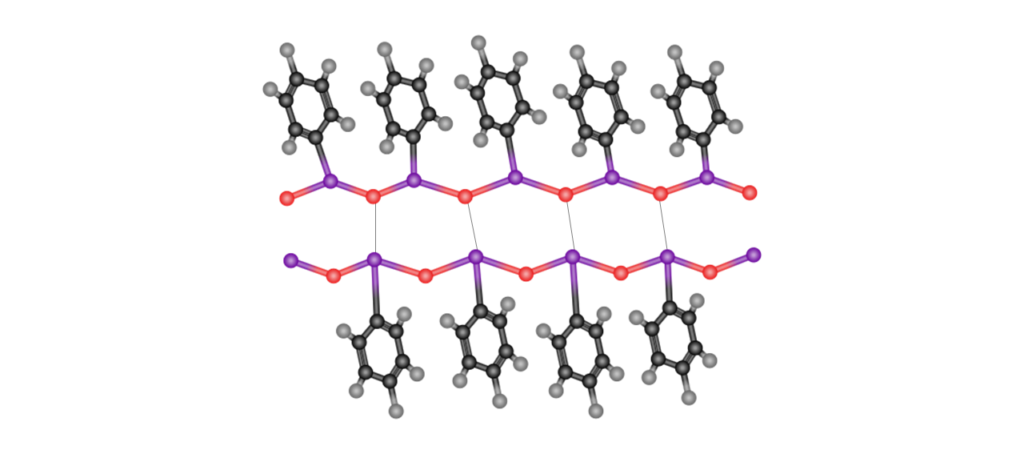
Iodosylbenzene forms higher-order association structures
Through aromatic ring and iodosyl group interactions, iodosylbenzene forms (C6H5I=O)n higher-order association structures, such as in the figure below.
Such structures cause poor solubility or insolubility in common organic solvents. However, introducing a sulfonic acid group into the ortho position will make iodosylbenzene soluble in water and methanol, and will also cause it to exhibit strong oxidation action. Iodosylbenzene also undergoes solvation in lower alcohols, where it exists as (dialkoxyiodo)benzene. Methanol and acetonitrile are commonly used as reaction solvents in reactions that use iodosylbenzene, including sulfide oxidation reactions and epoxy group synthesis.
Iodosyl compound reactions
Iodosylbenzene and other iodosyl compounds act as bases, and a protonic acid or Lewis acid can easily be added to convert it into an iodine (III) salt (Formula 1).
C6H5I=O + SO3 → C6H5I+OSO3– (Formula 1)
In hot water, iodosylbenzene will also undergo disproportionation into iodobenzene and iodylbenzene (Formula 2).
2 C6H5I=O → C6H5I + C6H5IO2 (Formula 2)
Iodosyl compounds are also used for the synthesis of iodine compounds. The following are examples of iodine compound synthesis using 2-iodosylbenzoic acid.
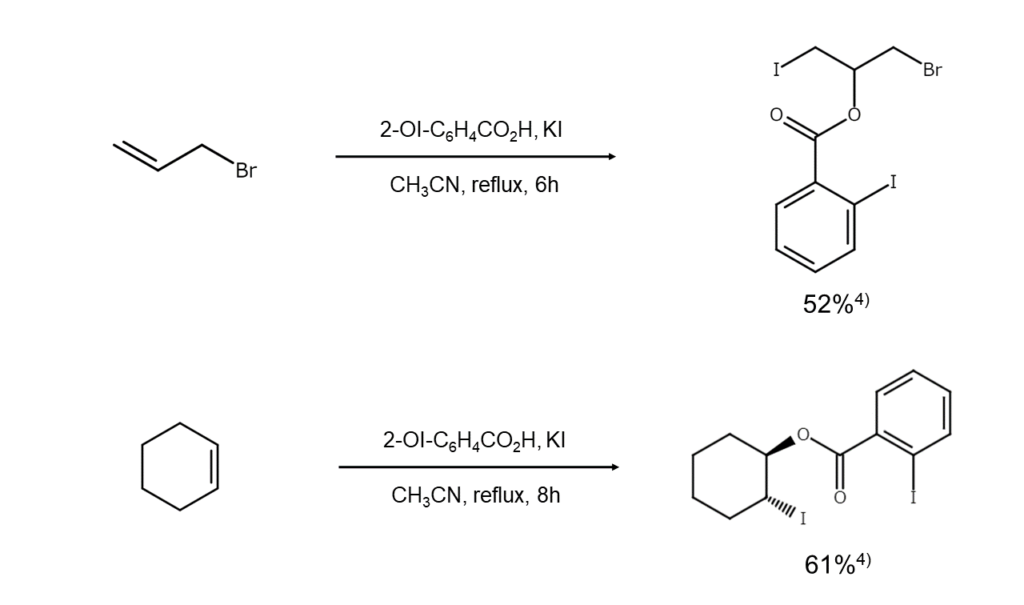
Introducing hypervalent organoiodine compounds: Iminoiodo compounds
Iminoiodo compounds as analogs of iodosyl compounds
Reacting a (diacyloxyiodo)arene with tosylamide under the presence of a base yields (N-tosyliminoiodo)arene, a nitrene analog of iodosylarene, as stable yellow crystals5) (Formula 3). Acyclic iminoiodo compounds such as these form higher-order association structures as a result of I···O and I···N intermolecular interactions, which means that these compounds do not dissolve well in common organic solvents.
ArI(OCOR)2 + 4-CH3C6H4SO2NH2 → ArI=NSO2C6H4CH3-4 (Formula 3)
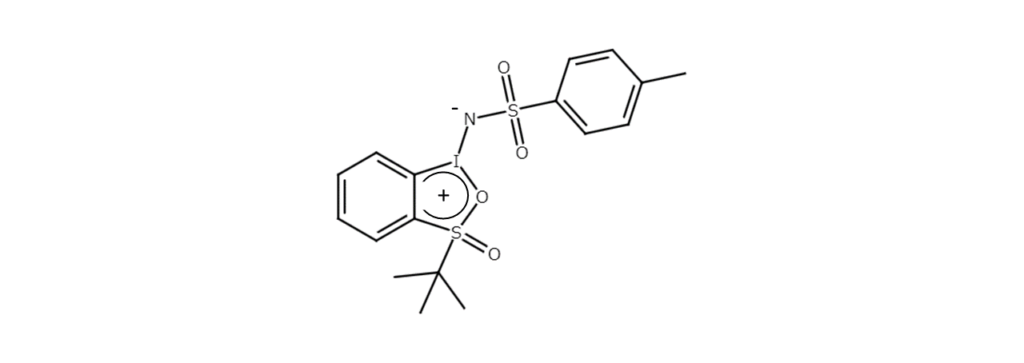
Conversely, cyclic iminoiodo compounds, such as the compound shown in the figure below, dissolve well in organic solvents. Phosphine and sulfide readily undergo imination and afford high yields of the corresponding heteroelement imine.6) This cyclic iminoiodo compound can be synthesized by reacting tosylamide with a (diacyloxyiodo)arene that has a bulky substituent in the ortho position.
Synthesis examples of other iminoiodo compounds
Reacting a (difluoroiodo)arene with N,N-bis(trifluoromethyl)benzenesulfonamide will yield the corresponding (imino)iodoarene as a relatively stable white solid (Formula 4). Taking the (imino)iodoarene and reacting it with phosphine will generate an iodo(imino)phosphorane, which will yield an iodosylbenzene when subjected to hydrolysis.
ArIF2 + C6H5SO2N(CF3)2 → ArI=NSO2C6H5 (Formula 4)
When (tetrafluoroiodo)benzene is reacted with N,N-bis(silyl)sulfonamide, a bis(iminoiodo)arene is produced (Formula 5). Bis(iminoiodo)arenes are white to slightly yellow crystalline powders that are unstable when exposed to light and are poorly soluble in common organic solvents. Further, bis(iminoiodo)arenes undergo hydrolysis with moisture, quantitatively giving iodylarenes.
ArIF4 + 2C6H5SO2N[Si(CH3)3]2 → ArI[=NSO2C6H5]2 (Formula 5)
Research Paper Review: “Structure and Reactivity of a Seven-Coordinate Ruthenium Iodosylbenzene Complex”
For this column, let’s find out what a 2023 research paper on iodosylbenzenes7) uncovered.
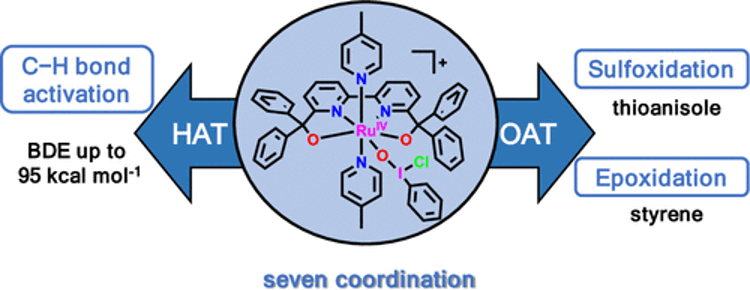
As indicated above, the research paper title is “Structure and Reactivity of a Seven-Coordinate Ruthenium Iodosylbenzene Complex.” The paper investigates the structure and reactivity of a seven-coordinate ruthenium iodosylbenzene complex, shown in the figure below.
(Source: Research Paper Page)
In recent years, complexes with iodosylarenes or other such compounds coordinated to metal ions have attracted attention as active oxidants. In response, this research paper reports on the synthesis of the complex illustrated in the figure above, and the investigation into aspects including the bond distance between each atom using X-ray structural analysis. The investigation determined that the complex features a distorted pentagonal bipyramidal geometry. It also revealed that the complex has exceedingly high reactivity and readily undergoes O-atom transfer (OAT) and C–H bond activation reactions with many different organic substances.
In the research paper abstract, the authors state, “This work should provide insights for the development of new highly reactive oxidizing agents based on CN7 geometry.”
We see here that iodosylbenzenes play important roles as reaction reagents in various forms. Iodosylbenzenes will certainly continue supporting many researchers alike as key compounds in organic chemical reactions.
MANAC offers manufacturing and marketing services for hypervalent organoiodine compounds. In particular, as we succeeded in significantly lowering our manufacturing costs of (diacetoxyiodo)benzene (DAIB), we can provide high-quality products at a low price.
Please feel free to inquire at the email address below.
chemia@manac-inc.co.jp
References
1) MANAC Inc. Research Lab., Suzuki, H. (ed.). Shuuso oyobi Youso Kagoubutsu no Yuuki Gousei: Shiyaku to Gouseihou [Organic Syntheses of Bromine & Iodine Compounds: Reagents & Synthesis Methods], Maruzen Publishing (2017).
2) Hossain, Md. D., Kitamura, T. Bull. Chem. Soc. Jpn., 2007, 80, 2213.
3) Koposov, A. Y., Karimov, R. R. et al. J. Org. Chem., 2006, 71, 9912.
4) Pan, Z., Liu, X. et al. Synthesis, 2005, 437.
5) Yamada, Y., Yamamoto, T. et al. Chem. Lett., 1975, 361.
6) Macikenas, D., Skrzypczak-Jankun, E. et al. J. Am. Chem. Soc., 1999, 121, 7164.
7) Pan, Y., Zhou, M. et al. Inorg. Chem. 2023, 62, 7772.













