(Diacyloxyiodo)arenes as effective gentle oxidants: Hypervalent organoiodine compounds (2): Discussion series on bromination/iodination reactions 30
Continuing from our previous article, we will discuss hypervalent organoiodine compounds.
Hypervalent organoiodine compounds have various applications, including as oxidants, dehydrogenating agents, and reagents for introducing functional groups. These compounds are gaining attention in recent years as environmentally friendly compounds that can be used as alternative reagents in place of toxic elements such as mercury and thallium.
In this article, we introduce (diacyloxyiodo)arenes, a specific hypervalent organoiodine compound type. These compounds, which are highly effective as gentle oxidants, also have an incredibly deep connection with MANAC. Be sure to read to the end as we share the full details of (diacyloxyiodo)arenes in this article.
contents
Hypervalent organoiodine compounds
Organic compounds that contain hypervalent iodine
A hypervalent organoiodine compound is just that — an organoiodine compound that is hypervalent.
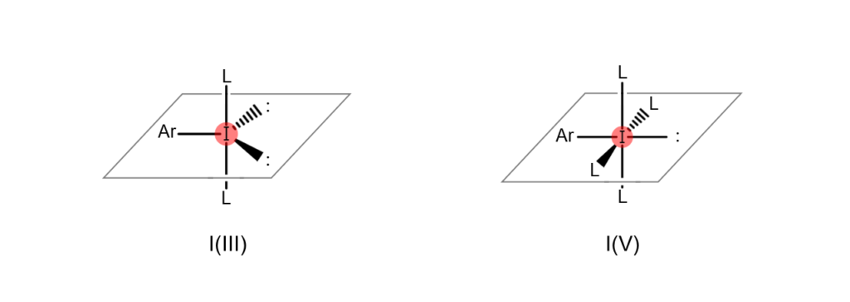
Atoms are “hypervalent” when the most outer electron shell (the part of the atom related to bonds with other atoms), which sits furthest from the nucleus, contains more than eight electrons. This state enables the atom to form a higher number of bonds with other atoms. For example, iodine atoms gain the ability to bond with multiple atoms at once when they become hypervalent, as shown in the figures below (the iodine atom on the left is trivalent, while the iodine atom on the right is pentavalent). When an organic compound contains hypervalent iodine, it is known as a “hypervalent organoiodine compound.”
Introducing hypervalent organoiodine compounds: (Diacyloxyiodo)arenes
Raw materials for synthesizing hypervalent organic compounds
(Diacyloxyiodo)arenes are compounds structured with an iodine atom bonded to one arene and two acyloxy groups. They can be synthesized by oxidizing an iodoarene with hydrogen peroxide or a peracid in a carboxylic acid solution. Synthesis methods that acylate an iodosylarene using an acid anhydride are also known.
As stable compounds that are easy to work with, (diacyloxyiodo)arenes are valuable as raw materials to synthesize hypervalent organoiodine compounds. (Diacyloxyiodo)arenes are also used as mild oxidants. They offer the advantage of being storable considerably long term without decomposing as long as the storage conditions are cool, dark, and dry.
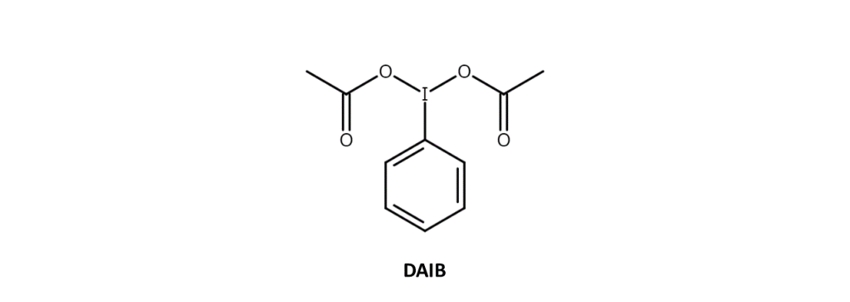
One of the most prominent (diacyloxyiodo)arenes is DAIB, shown below. “DAIB” is an abbreviation for (diacetoxyiodo)benzene, which is also known alternatively as iodobenzene diacetate and phenyliodine diacetate (PIDA). DAIB features the simplest structure among the (diacyloxyiodo)arenes and is used extensively as a reagent and a raw material for synthesis.
To learn more about DAIB, be sure to check the article below.
(Diacyloxyiodo)arene synthesis
A great variety of synthesis methods can be used to synthesize (diacyloxyiodo)arenes. Here are a couple of examples of such reactions.

Iodination reactions with (diacyloxyiodo)arenes
(Diacyloxyiodo)arenes can be used as reagents to iodinate various compounds. This time, let’s take a look at two iodination methods that use (diacyloxyiodo)arenes.
① Methods using tetra-alkylammonium iodides
Reacting a (diacyloxyiodo)arene with a tetra-alkylammonium iodide will generate an ammonium salt of diacyloxyiodate (I), as shown below.
C6H5I(OCOCH3)2 + (C2H5)4N+I– → [(C2H5)4N]+[I(OCOCH3)2]– + C6H5I
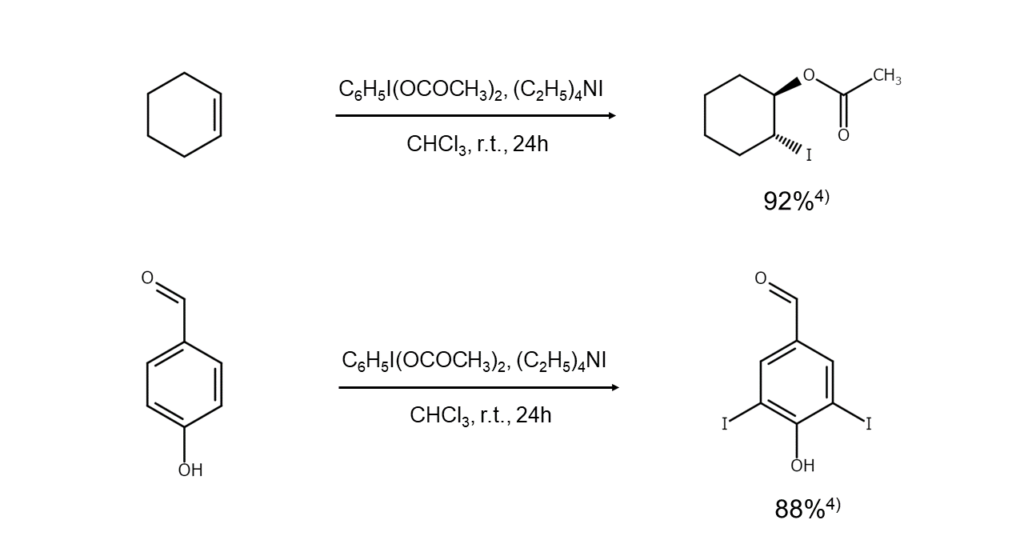
These ammonium salts are structurally analogous to Simonini complexes (AgI(OCOCH3)2), and because acyl hypoiodite is generated when they decompose, these ammonium salts also act as iodinating agents.4) Here are examples of these reactions.
② Methods using active methylene compounds
Reacting a (diacyloxyiodo)arene with a carbanion generated from an active methylene compound, such as 1,3-diketone, beta-ketoester, or malononitrile, will produce a stable iodonium ylide, such as in the reaction figure below.5) Reacting this ylide with NBS or NIS yields a gem-dihalogeno compound.
C6H5I(OCOCF3)2 + K+[CH(CO2CH3)2]– → [C6H5I]+[C(CO2CH3)2]– + CF3CO2K + CF3CO2H
Column: Do hypervalent compounds that contain bromine exist?
The hypervalent iodine compounds that we have covered since the last issue are exactly as they sound — hypervalent compounds that contain iodine. This may have some wondering about whether there are hypervalent compounds that contain another important element in this series: bromine.
Interestingly, research on hypervalent bromine compounds has hardly progressed compared to their iodine counterparts. A major factor behind this is bromine’s larger ionization potential over iodine (e.g., iodobenzene: 8.69 eV; bromobenzene: 8.98 eV) and the notorious difficulty in oxidizing monovalent bromine into a trivalent or pentavalent state.
Be that as it may, bromine as a raw material is more readily available than iodine, and forgoing its many advantages would constitute a missed opportunity of sorts. Considering the ionization potential of bromine, hypervalent bromine compounds are expected to exhibit higher reactivity than hypervalent iodine compounds. With such great promise, research is ongoing worldwide in a hopeful pursuit of hypervalent bromine compounds to use in the field of organic chemistry.
For example, in 2021, a research group from Chiba University in Japan announced their success in developing a chiral hypervalent bromine compound, a world-first achievement. The compound is anticipated to enable the efficient synthesis of pharmaceutical raw materials.
*Check here for the press release from the research group at Chiba University (available only in Japanese):
https://www.chiba-u.ac.jp/general/publicity/press/files/2021/20211207_1.pdf
While they remain overshadowed by hypervalent iodine compounds, hypervalent bromine compounds have tremendous hidden potential. We highly recommend those interested to take a further look into this topic.
MANAC offers manufacturing and marketing services for hypervalent organoiodine compounds. In particular, as we succeeded in significantly lowering our manufacturing costs of (diacetoxyiodo)benzene (DAIB), we can provide high-quality products at a low price.
Please feel free to inquire at the email address below.
chemia@manac-inc.co.jp
MANAC’s high-quality, low-cost DAIB is an achievement borne of the passionate dedication of the researchers at MANAC. Find out more about the story of MANAC researchers and their unwavering persistence in the face of countless failures during development.
References
1) MANAC Inc. Research Lab., Suzuki, H. (ed.). Shuuso oyobi Youso Kagoubutsu no Yuuki Gousei: Shiyaku to Gouseihou [Organic Syntheses of Bromine & Iodine Compounds: Reagents & Synthesis Methods], Maruzen Publishing (2017).
2) Sharefkin, J. G., Saltzman, H. Org. Synth. Coll. Vol. V, 660 (1973).
3) Hossain, Md. D., Kitamura, T. Synthesis, 2005, 1932.
4) Doleschall, G., Gabor, T. Tetrahedron, 1980, 36, 1649.
5) Neimanis, D., Neiland, O. Zh. Org. Khim., 1970, 6, 633.