Highly selective yet gentle brominating agents: N-bromo compounds (1): Discussion series on bromination/iodination reactions 1
Bromination and iodination reactions sit at the core of MANAC’s technological capabilities. The bromine and iodine compounds manufactured by MANAC are used as chemical intermediates in a large number of areas, including in pharmaceuticals, agrochemicals, and semiconductor materials.
However, even though bromination and iodination reactions play a crucial role in organic syntheses, there exists little material or literature that discuss these reactions systematically. This lack of available information has surely led many companies to experience challenges when conducting synthesis reactions.
In response, we are launching a Chemia discussion series on MANAC’s bromination and iodination technologies. This first article of the series covers N-bromo compounds, which are commonly used brominating agents. In addition to discussing basic information regarding N-bromo compounds, such as characteristics, benefits, and usage precautions, this article also covers some of MANAC’s use case examples.
■ What you can learn from this article ✔ N-bromo compounds are utilized as gentle and highly selective brominating agents in a variety of reactions. ✔ Bromination reactions are based on a radical mechanism, and reaction conditions may lead to isomerization or side reactions. ✔ At MANAC, various brominating agents are employed to select the most suitable one for efficient synthesis. ■ Recommended Articles ・ A first-line brominating agent:describing N-bromosuccinimide (NBS): N-bromo compounds (2): Discussion series on bromination/iodination reactions 2 ・ A fascinating brominating agent capable of minimizing costs and byproducts, describing 1,3-dibromo-5,5-dimethylhydantoin (DBDMH): N-bromo compounds (8): Discussion series on bromination/iodination reactions 8 ・ A brominating agent that facilitates addition reactions with alkenes, overview and reaction mechanisms of N-bromoacetamide (NBA): N-bromo compounds (10): Discussion series on bromination/iodination reactions 10 ・ One of the most powerful brominating agents, overview and reaction mechanisms of dibromoisocyanuric acid (DBI): N-bromo compounds (11): Discussion series on bromination/iodination reactions 11
contents
N-bromo compounds
highly selective yet gentle brominating agents
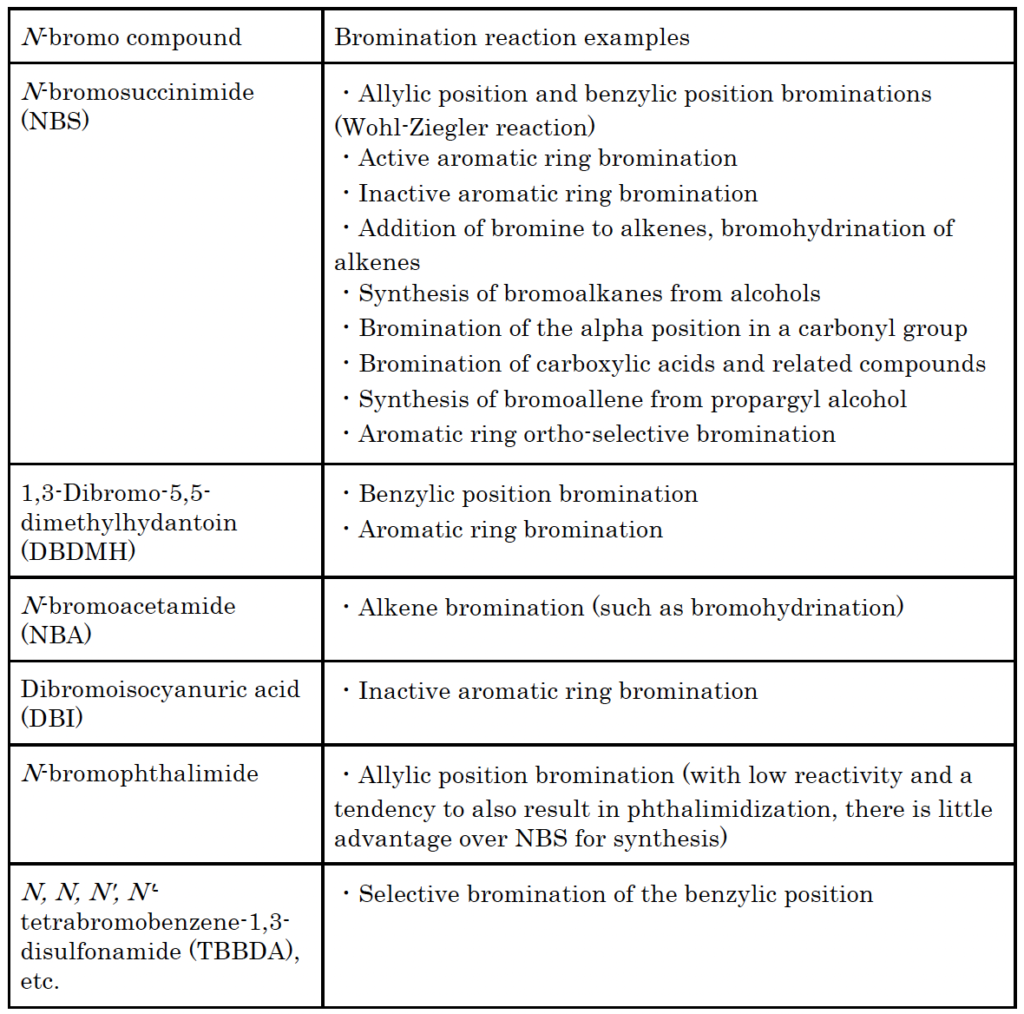
N-bromo compounds contain a bromo group (-Br) on a nitrogen atom. N-bromoimide and N-bromoamide are examples of N-bromo compounds, and are commonly used in the laboratory as highly selective yet gentle brominating agents. Major N-bromo compounds are listed below
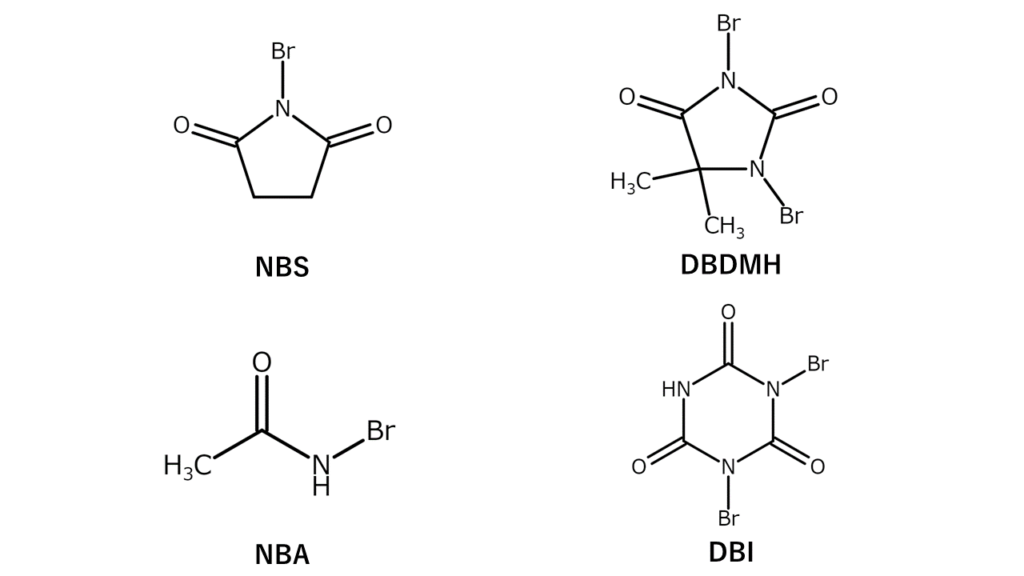
These N-bromo compounds are powders and carry the advantage of being easier to handle quantitatively compared to bromine, a highly volatile liquid. However, they also have certain disadvantages, including that they leave large amounts of imides and amides after reactions, and that they suffer from poor atom economic efficiency (which refers to the percentage of atoms used effectively without waste in a reaction).
N-bromo compound selectivity
N-bromo compounds are useful in selectively brominating activated positions in unsaturated bonds (such as the allylic position, the benzylic position, and in carbonyl groups, the alpha position). However, additions to double bonds can occur depending on the reaction conditions, resulting in the bromination of other positions.
Bromination reactions using N-bromo compounds
The principle of bromination
Let’s consider a bromination reaction at the allylic position using N-bromosuccinimide (NBS). When the N-Br bond present in NBS is radically cleaved, the resulting bromine radicals extract the hydrogen atoms bonded to the carbon at the allylic position, forming allyl radicals and HBr. This HBr then reacts with different NBS, forming Br2 in situ. The Br2 reacts with the allyl radicals mentioned just earlier, brominating the allylic position. Because additional bromine radicals are generated in the bromination process, a radical chain reaction occurs.
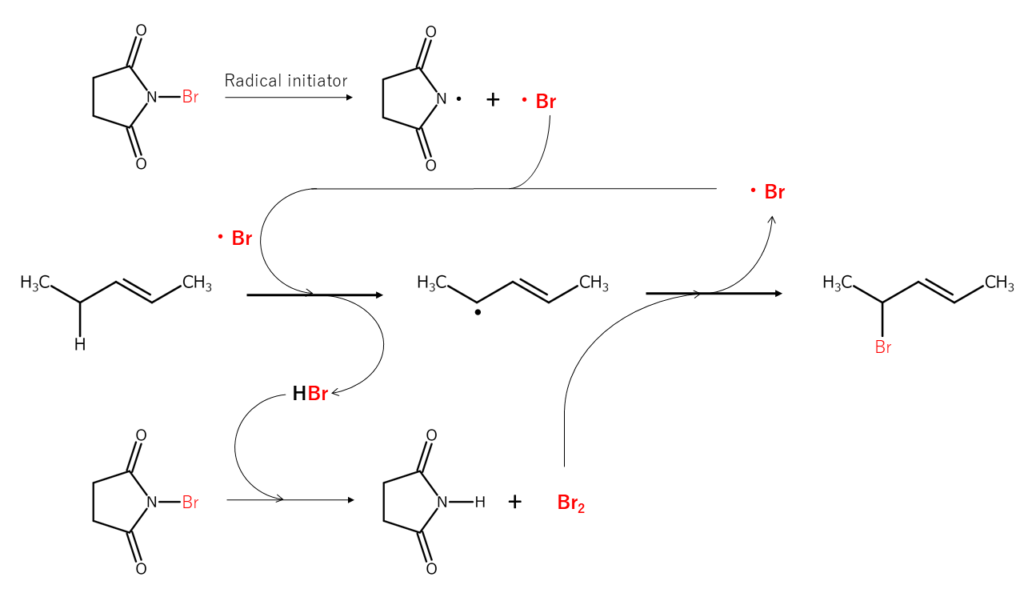
The bromination reactions shown above are known to typically occur in the following order: CH2 (secondary carbon) > CH3 (primary carbon) > CH (tertiary carbon). In many cases, the bromination of tertiary carbon requires the addition of peroxides or the application of photo-irradiation.
Precautions regarding bromination reactions
The following precautions must be followed when carrying out bromination reactions using N-bromo compounds.
・It is crucial to pay attention when selecting reaction conditions as isomerization or HBr desorption may readily occur depending on the structure of the resulting product.
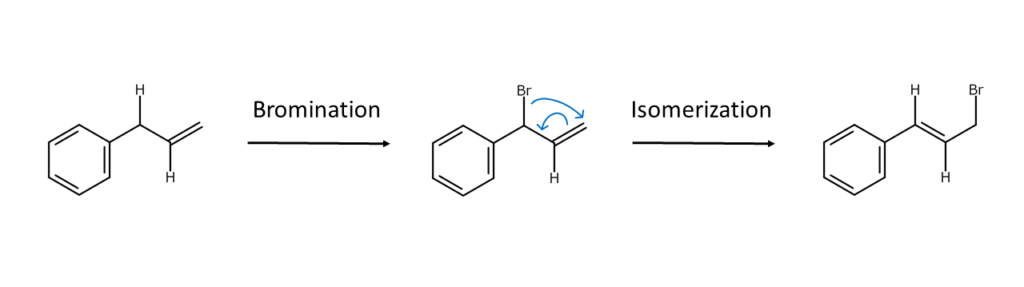
・N-bromo compounds may react violently with organic compounds when peroxides are also present.
・Depending on the person, contact with N-bromo compounds may result in skin irritation or rashes.
Examples of common bromination reactions using N-bromo compounds
N-bromo compounds are used for the bromination reactions below.
N-bromo compounds are not always the first go-to at MANAC
Since NBS and DBDMH are powders and thus easy to work with, they are often used as first-line brominating agents at research institutions such as universities. At MANAC, however, N-bromo compounds are not always the first go-to for brominating agents. Examples of when N-bromo compounds are used at MANAC include cases in which the ratio between the raw material and the brominating agent need to be controlled precisely, and cases in which the side reactions caused by brominating agent byproducts are an issue.
So why are there many cases in which N-bromo compounds are not used for bromination reactions at MANAC? The secret behind this lies within MANAC’s factory facilities. MANAC’s factories are strung with a network of bromine pipelines, providing access to bromine for each factory location. Besides the easily handled N-bromo compound powders, this network of pipelines enables the use of many different types of brominating agents. The ability to select the best brominating agent for each reaction is one of MANAC’s major strengths.
MANAC manufactures and sells NBS and DBDMH, two common N-bromo compounds. Please feel free to inquire.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing
2) Braude, E. A., Waight, E. S. J. Chem. Soc., 1952, 1116.