Iodoalkane synthesis: Iodination reactions with halogen exchange (2): Discussion series on bromination/iodination reactions 13
Iodides are used as intermediates in many fields, including active pharmaceutical ingredients (APIs), antibacterial agents, and electronic materials. An essential aspect behind synthesizing these iodides is the halogen exchange reaction.
Halogen exchange is used as a method of indirect iodination for compounds that do not easily undergo direct iodination. The relatively large number of substrates compatible with this useful method has lent to a wide range of applications. Basic knowledge of halogen reactions is fundamental for learning about iodination reactions.
This article looks at iodoalkane synthesis reactions, which can be described as synonymous with halogen exchange iodination. With detailed explanations of reaction mechanisms and example reactions, this article is geared toward providing readers with helpful information for research activities.
■ What you can learn from this article ✔ In the Finkelstein reaction, NaI or KI is commonly used as the reagent; however, MgI₂ or CaI₂ may also be used to increase the concentration of iodine ions. ✔ Because of their low reactivity, fluoroalkanes generally do not react easily with typical alkali iodides. However, using iodotrimethylsilane makes it easier to obtain iodoalkanes. ✔ Diiodomethane is used not only in density measurements for minerals but also as a reaction reagent that cyclopropanates alkenes, making it useful to API manufacturers. ■ Recommended Articles ・ Acyl iodide synthesis, iodoarene synthesis: Iodination reactions with halogen exchange (3): Discussion series on bromination/iodination reactions 14 ・ Iodoalkane synthesis from alcohols: Iodination reactions using alkali iodides (2): Discussion series on bromination/iodination reactions 25 ・ Iodoalkane synthesis from ether cleaving and other syntheses using alkali iodides: Iodination reactions using alkali iodides (3): Discussion series on bromination/iodination reactions 26
contents
Describing halogen exchange
A method for converting chlorides and bromides into iodides
Halogen exchange is a method that replaces a halogen atom in a compound with a different halogen atom. In this case, the halogen exchange method replaces a chlorine atom or a bromine atom with an iodine atom.
There are several types of halogen exchange reactions. Among these, the halogen exchange reaction most commonly used for iodination reactions is the Finkelstein reaction2, which replaces a halogen atom with a different halogen atom through a bimolecular nucleophilic substitution reaction (SN2 reaction). The reaction formula is shown below.
R-X + NaI ⇌ R-I + NaX X = Cl, Br
Because of its wide-ranging substrate applications, the Finkelstein reaction has long been used to synthesize iodoalkanes and other products. See this article for details on reaction principles.
Halogen exchange iodination reactions: Iodoalkane synthesis
Reaction details
In this article, we discuss reactions that synthesize iodoalkanes through halogen exchange. Since fluoroalkanes generally have low reactivity, chloroalkanes and bromoalkanes are usually used as reactants. Details of these reactions are as follows below.
(1) Using chloroalkanes or bromoalkanes as reactants
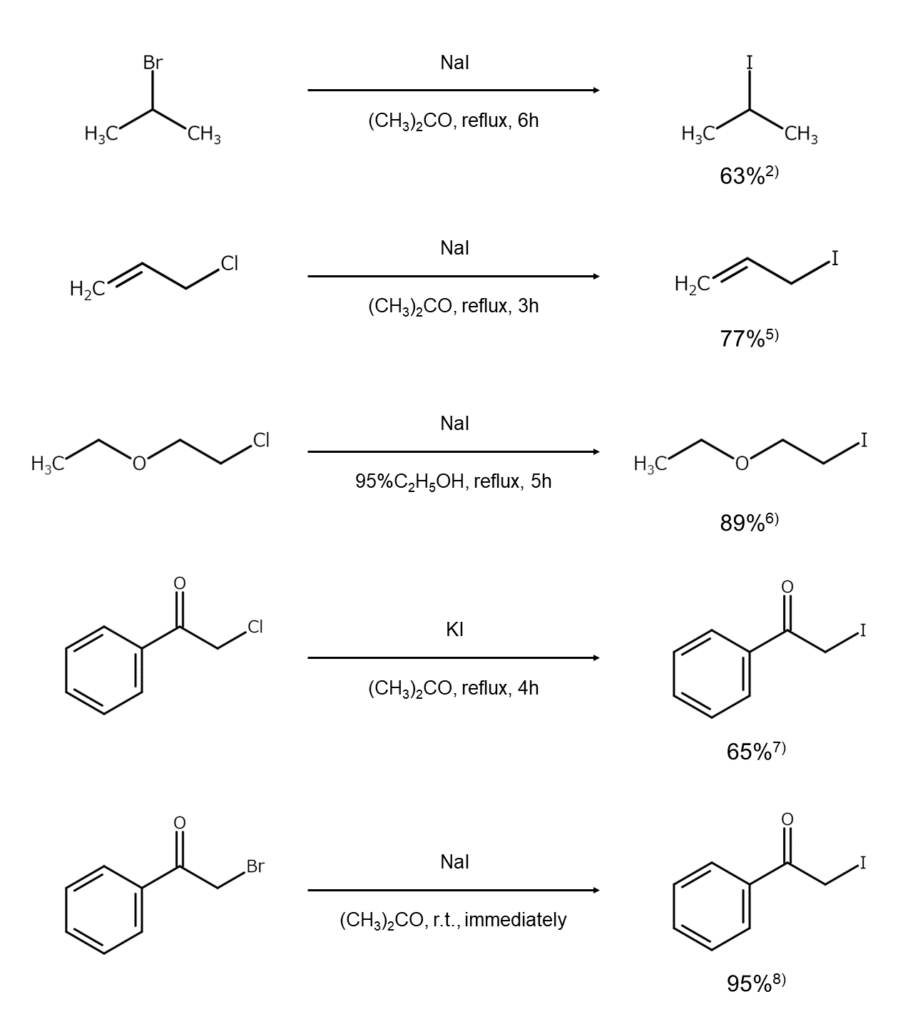
Methods that use the Finkelstein reaction are standard for chloroalkanes and bromoalkanes. In these reactions, a chloroalkane or bromoalkane reacts with an alkali iodide in a polar solvent, such as acetone, methanol, or acetonitrile2-4. NaI and KI are common reagents for these reactions, but when aiming for higher concentrations of iodine ions, MgI2 or CaI2 may be used instead.
Examples of iodoalkane synthesis through the Finkelstein reaction are shown below.
The following are examples of other known halogen exchange reactions.
・Halogen exchange reactions with tertiary alkyl halides and other such compounds
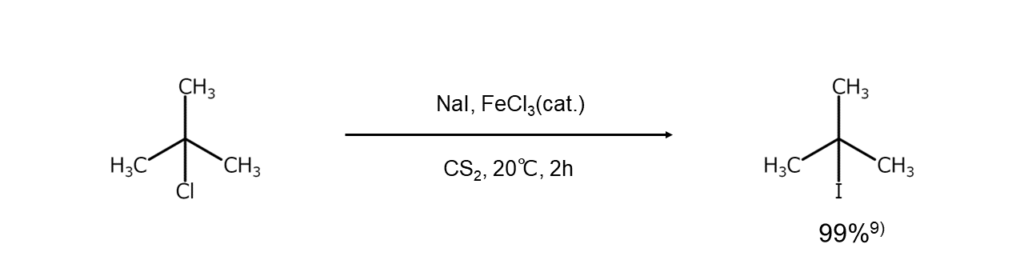
When using tertiary alkyl halides, α-bromoketones, or α-bromocarboxylic acids as reactants, halogen exchange reactions may be carried out by heating the reactants together with hydroiodic acid under a reflux or by heating them with KI in acetic acid. However, caution is required regarding reaction conditions because the halogen atoms in tertiary alkyl halides may be removed reductively after being replaced by iodine atoms.
As shown below, a method has also been reported where iron chloride (III) acts as a catalyst when conducting reactions with tertiary alkyl halides in carbon disulfide.
・Halogen exchange reactions using organometallic compounds
For compounds with halogen atoms that do not readily undergo direct exchange with iodine atoms, a method may be used to first convert an alkyl halide into a Grignard reagent or an organic lithium compound before processing it with iodine (indirect halogen-iodine exchange reaction).
While this method is suitable for synthesizing high-purity iodoalkanes, it incurs high costs. Therefore, its actual use is generally limited to the synthesis of special iodine compounds.
・Halogen exchange reactions through exposure between gaseous phase and solid phase materials
This method exposes chloroalkane or bromoalkane vapors to ammonium or a phosphonium iodide in order to bring about halogen exchange.
(2) Using fluoroalkanes as reactants
As the reactivity of fluoroalkanes is generally low, these compounds do not readily react with alkali iodides. However, when iodotrimethylsilane, with the chemical formula (CH3)3SiI, is set to act on a fluoroalkane under a moderate homogeneous system, an exchange of fluorine atoms and iodine atoms occurs, enabling a high iodoalkane yield. This reaction is thought to progress via a pentacoordinate silicon intermediate, as shown below.
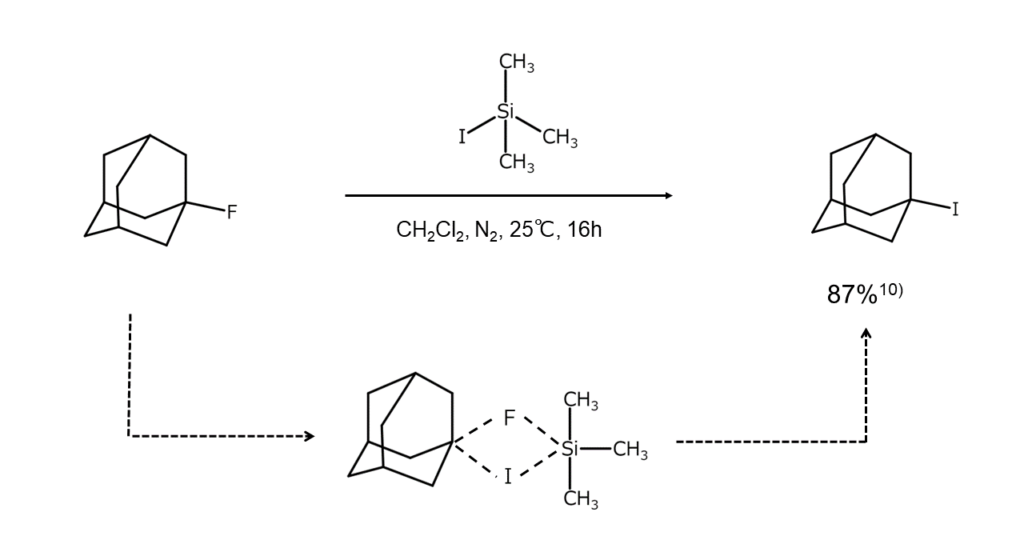
The above reaction readily proceeds when using a tertiary fluoroalkane as a reactant. However, when using primary and secondary fluoroalkanes, the reaction is reportedly sluggish and tends to result in isomerization. This method can also be used with tertiary chloroalkanes10.
As an alternative to iodotrimethylsilane, equivalent results can be achieved with reagents formed in situ from hexamethyldisilane ([(CH3)3Si]2)-iodine or in situ from chlorotrimethylsilane ((CH3)3SiCl)-NaI.
Diiodomethane in pharmaceuticals and mineral analysis
The halogen exchange reactions covered in this article can also be used to synthesize diiodoalkanes, which contain two iodine atoms in each molecule. Diiodoalkane compounds are vital to many different fields.
The following are application examples of diiodomethane, the simplest of the diiodoalkanes.
One application of diiodomethane is mineral analysis. Diiodomethane has a relatively high density (3.325 g/cm3), so it can be used in density measurements and heavy-media separation for minerals and other solid materials.
Diiodomethane is also used as a reaction reagent, enabling the cyclopropanation of alkenes, such as in the reaction below (Simmons-Smith reaction).

Cyclopropane skeletons such as these can be found in many APIs. These cyclopropane skeletons play a crucial role in effectively bonding APIs with target proteins. For this reason, API manufacturers utilize diiodomethane to form cyclopropane skeletons in API compounds.
At MANAC, our business includes comprehensive, one-stop commissioned services for API manufacturers, from the provision of diiodomethane to the conduction of cyclopropanation reactions, as well as the collection and recycling of resulting iodine byproducts. Find out more in the article below.
References
1) MANAC Inc., Research Laboratory, Suzuki, Hitomi (ed.), “Organic Syntheses of Bromine & Iodine Compounds”. Maruzen Publishing. (Japanese)
2) Finkelstein, H. Ber. 1910, 43, 1528.
3) Tundo, P., Venturello, P. Synthesis, 1979, 952.
4) Clark, J. H., Jones, C. W. et al. J. Chem. Res., 1989, 238.
5) Letsinger, R. L., Traynham, J. G. J. Am. Chem. Soc., 1948, 70, 2818.
6) Swallen, L. C., Boord, C. E. J. Am. Chem. Soc., 1930, 52, 651.
7) Bordwell, F. G., Brannen, jr., W. T. J. Am. Chem. Soc., 1964, 86, 4645.
8) Rheinboldt, H., Perrier, M. J. Am. Chem. Soc., 1947, 69, 3148.
9) Miller, J. A., Nunn, M. J. J. Chem. Soc. Perkin Trans. 1, 1976, 416.
10) Olah, G. A., Narang, S. C. et al. J. Org. Chem., 1981, 46, 3727.